The feminine mystique is not just figurative—it also extends to women’s reproductive anatomy. For decades women were excluded from research studies, leading to a dearth of information about female physiology that is only just starting to be filled in. Some insights have come from research on tissue grown in standard petri dishes but these studies still cannot represent the intricacies of a woman’s menstrual cycle.
Now in a bioengineering first, researchers have created a miniature laboratory model of the entire female reproductive tract, complete with hormone signaling. This 3-D “organ-on-a-chip” system may improve our understanding of the causes of recurrent miscarriage and fuel new research into birth control and other drug development. The work may also inch medicine toward a future when fertility experts could simply grow a sample of an individual woman’s cells, place them in this chip system and determine the best treatment.
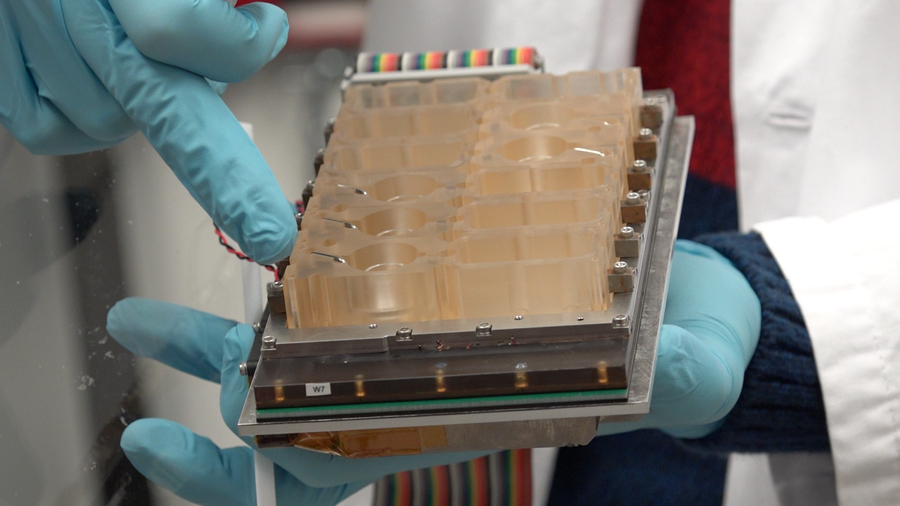
EVATAR is a 3-D model of the female reproductive tract, capable of mimicking the subtle hormonal interplay of the menstrual cycle. Credit: Northwestern Medicine
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
To model the female reproductive system, a team of researchers led by Northwestern University’s Teresa Woodruff took lab-grown human and mouse cells from five organs and cultivated them in a network of interconnected cubes. The cubes were fed by tubes that allowed blood and hormones to flow through them, mimicking the fluids’ movement throughout the body. Valves and pumps also controlled the units’ pressure and airflow. This environment allowed cells that would normally die in a petri dish—suffocating amid their own cellular waste—to stay alive for a standard 28-day reproductive cycle.
After the researchers jump-started the system’s hormonal communication with an injection of pituitary hormone, the cells secreted levels of estrogen and progesterone found in a typical menstrual cycle and the signaling that occurs between female reproductive organs. The team was also able to simulate hormone levels during ovulation as well as the early stages of pregnancy, creating a tool that could potentially yield insight into how to maintain successful pregnancies. The feats are described today in Nature Communications. “This represents not only a revolution in cell culture technique [but also] an evolution of the study of the reproductive tract and disease,”Woodruff says.
The menstrual-cycle-on-a-chip system includes mouse ovarian cells, along with human cells from the fallopian tube, endometrium and cervix obtained from hysterectomies. (Human ovarian cells were not available, but mouse ovarian cells produce the same hormones.) The system also includes human liver cells, included because that organ breaks down many drugs. The work builds on earlier efforts by Linda Griffith and colleagues at Massachusetts Institute of Technology, funded by the Defense Advanced Research Projects Agency, to develop a “liver on a chip.” The menstrual chip research team significantly expanded on that technology and those of many other groups to produce the current design for modeling reproductive cycles.
The new chip system is far from a perfect stand-in for female anatomy: “Right now organs-on-a-chip cannot account for something like an early-life [toxic] exposure that might affect future reproductive health,” says Kevin Osteen, a professor obstetrics and gynecology at Vanderbilt University School of Medicine who was not involved in the study but works on other reproductive chip models. The new chip system also does not include the placenta, which is key to supporting pregnancy, nor does it factor in how inflammation due to a viral infection would affect reproductive organs. Still, Woodruff says, her system opens possibilities for studying a wide range of conditions, such as diseases of the cervix, which cannot be modeled in mice because their cervical cells are completely different from the human variety. She adds, “This system will allow us to study infection in that organ in way we haven’t been able to do in the past.”