Mark Denison began hunting for a drug to treat COVID-19 almost a decade before the contagion, driven by a novel coronavirus, devastated the world this year. Denison is not a prophet, but he is a virologist and an expert on the often deadly coronavirus family, members of which also caused the SARS outbreak in 2002 and the MERS eruption in 2012. It is a big viral group, and “we were pretty certain another one would soon emerge,” says Denison, who directs the division of pediatric infectious diseases at Vanderbilt University Medical Center.
A virus is an unusual beast. Essentially it is a cluster of genetic material that integrates itself into a cell and takes over some of the cell’s molecular machinery, using it to assemble an army of viral copies. Those clones burst out of the cell, destroying it, and go on to infect nearby cells. Viruses are hard to kill off completely because of their cellular integration—they hide within their hosts. And they have explosive reproductive rates. Because total eradication is so hard, antiviral drugs instead aim to limit replication to low levels that cannot hurt the body.
In 2013 Denison and Ralph Baric, a coronavirus researcher at the University of North Carolina at Chapel Hill, identified a vulnerable site on a protein common to all coronaviruses they had examined, a spot that is key to the microbe’s ability to make copies of itself. If that ability is hindered, a coronavirus cannot cause widespread infection. Four years later researchers in the two laboratories spotted a compound that acted on this protein site. It was sitting, unused, in a large library of antiviral compounds created by the biotech giant Gilead Biosciences. The scientists got a sample and, in test tube and animal experiments, showed that the drug, called remdesivir, shut down the replicating machinery of several coronavirus variants.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
So in early January, when the alarms rang about SARS-CoV-2, Denison and Baric alerted colleagues at Gilead that they were sitting on a potential treatment. Largely because of its activity against other coronavirus strains in Denison and Baric’s animal studies, remdesivir was made available to patients for “compassionate use” in January. By March, Gilead had rushed the compound into two human trials, planning to test the drug’s safety and most effective doses on about 1,000 ill patients over several months; health authorities in China began two similar trials. While that was happening, Denison, Baric and a group of their colleagues at Emory University identified still another compound, called EIDD-2801, that hits the same viral vulnerability. In early April they published results showing that in mice, the new substance helped breathing and reduced the amount of many coronaviruses. In test-tube experiments with human lung cells, it drastically hindered SARS-CoV-2.
Several labs around the world, like Denison’s and Baric’s, have logged years of experience poking about the inner workings of coronaviruses because of SARS and MERS. By the time the new coronavirus was genetically sequenced and its structure revealed, scientists already had identified the enzymes and proteins that most coronaviruses use to spread from one infected human cell to another and also understood that the body could create an overly aggressive inflammatory response when the virus infected lung airway cells.
Because of this work, three main strategies for impeding the virus have emerged as the labs have turned to the current threat. One strategy is to find compounds like remdesivir and EIDD-2801 that gum up the virus’s reproductive machinery when it enters a target cell. A second is to block the virus, like a bouncer outside a bar, from entering and infecting those cells in the first place. The third approach is to muffle the immune system’s dangerously overactive response, a “cytokine storm” that can drown a victim in a mass of congestion and dying airway cells.
To find these drugs, researchers have turned to the Food and Drug Administration’s list of some 20,000 compounds approved for human use and crawled through drug patent applications looking for compounds with promising mechanisms of action. The goal has been to find drugs that have been at least partly developed, avoiding years of making therapeutic molecules from scratch. The Milken Institute, a health advocacy think tank, counted 133 experimental COVID-19 treatments in mid-April. About 49 of these therapies are being rushed into clinical trials. Their effectiveness in people is not yet known, and scientists caution that such drugs, like other antivirals, are unlikely to be cures. But they could reduce symptoms enough to give patients’ immune systems a chance to beat the virus on their own.
COPY STOPPERS
All coronaviruses use the same mechanism to reproduce, which involves an enzyme called viral RNA polymerase, so Baric says that was an obvious target. The polymerase makes lots of mistakes as it copies the virus, and it relies on another enzyme, known as an exonuclease, to “proofread” and fix them. Remdesivir appears to disable the proofreading enzyme. Then the virus’s copying factory becomes sloppy and produces fewer new viruses.
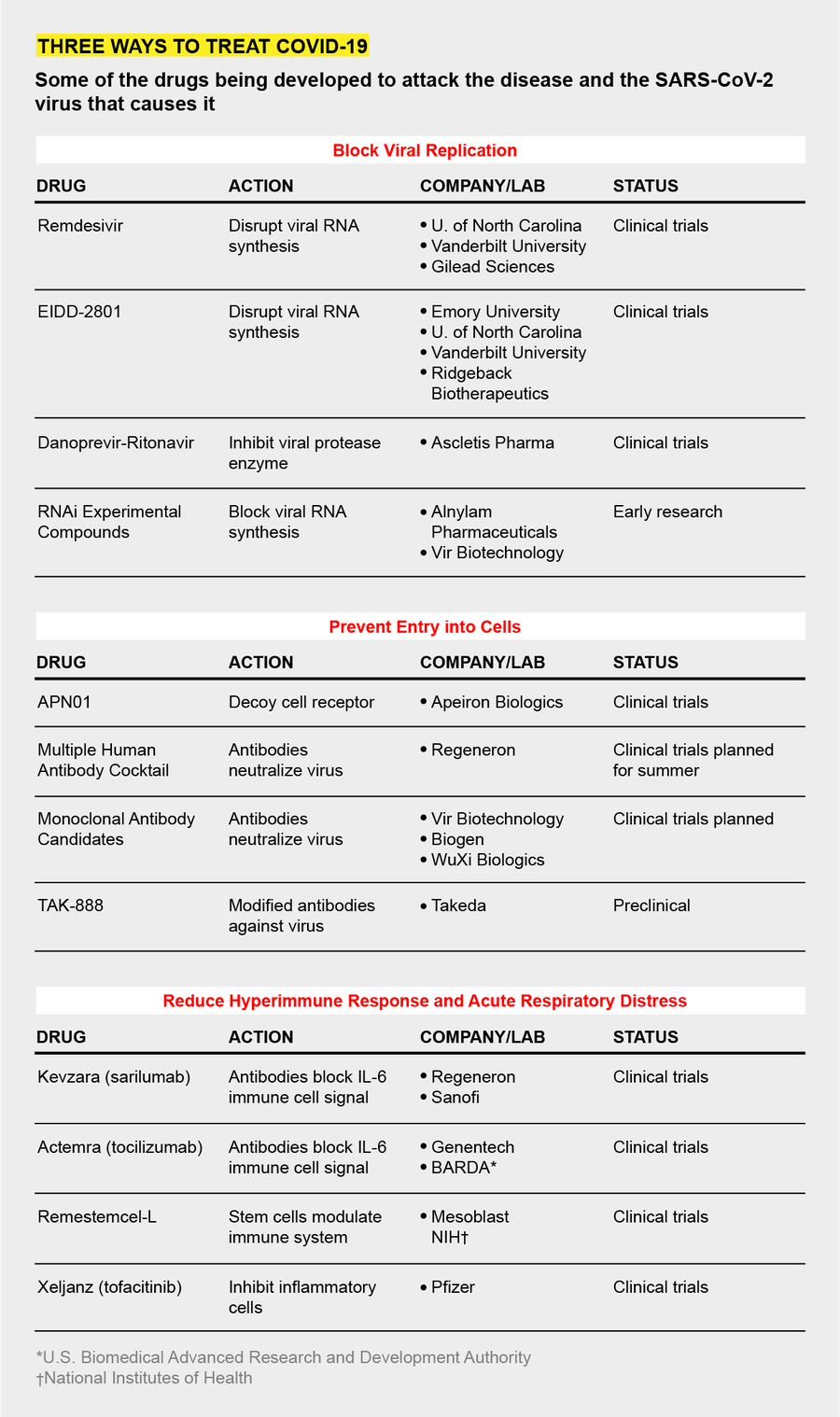
EIDD-2801, the compound with promising animal and test-tube results reported in early April, aims at the same viral enzyme. But unlike remdesivir, which much be given intravenously, EIDD-2801 can be taken as a pill. For this reason, Baric and other researchers investigating EIDD-2801, including George Painter, a professor of pharmacology and president of the Emory Institute for Drug Development, which first produced the drug, suspect it may end up being more widely used than remdesivir.
In 2018 Painter and his colleagues identified EIDD-2801’s activity during a search for a universal influenza medicine. When SARS-CoV-2 emerged, Painter’s group immediately shifted focus. EIDD-2801, like remdesivir, inhibits the coronavirus’s self-copying operations, but it also works against virus variants with a mutation that made them resistant to the Gilead drug. In addition, EIDD-2801 is effective against a host of other RNA viruses, so it could serve as a multipurpose antiviral, much as some antibiotics can work against a wide variety of bacteria. For COVID-19, says Wayne Holman, co-founder of Miami-based Ridgeback Biotherapeutics, which has licensed the drug and is planning clinical trials, the goal is to have a pill that can be taken by patients at home early in the course of the disease to prevent it from progressing.
BLOCKING INFECTION
To stop SARS-CoV-2 from penetrating cells in the first place, scientists are trying to develop antibodies that lock onto the viral protein that facilitates cell entry, a part of the virus known as the spike. Some of these neutralizing antibodies, made of a protein called immunoglobulin, may come from the blood of patients who have already cleared the virus. Several medical centers, including Johns Hopkins Hospital and the Mayo Clinic, are harvesting blood plasma from survivors and screening it for antibodies. In a technique known as convalescent therapy, doctors then transfuse it into hospitalized patients with life-threatening acute respiratory distress. Early studies of a few such patients suggest the approach may work—some patients’ symptoms improved, and levels of the virus in their bodies dropped—but the work is very preliminary.
Takeda Pharmaceuticals, a Japanese firm, is also collecting plasma from recovered COVID-19 patients to identify antibodies. In that plasma, the company is identifying antibodies that show the most activity against SARS-CoV-2. Using these antibodies as a template, the Takeda researchers plan to synthesize a batch of even more active versions to create a potent cocktail of infection inhibitors, says Chris Morabito, head of research and development of plasma-derived therapies. The therapy—TAK-888—might enter clinical trials by year’s end, Morabito says; the number “888” represents “triple fortune” in Chinese. Several other drugmakers, including Regeneron and Vir Biotechnology, are generating their own therapeutic antibodies and say they will also be tested in patients this year.
Another blockade strategy focuses on the cellular docking site that the virus uses. Josef Penninger, a molecular biologist at the University of British Columbia in Vancouver and founder of drug company Apeiron Biologics, is trying to lure the virus away from a chemical receptor called ACE2 in the outer wall of lung cells. The coronavirus spike protein binds to this receptor. Several years ago Penninger’s lab synthesized a decoy version of ACE2. In test-tube experiments, the scientists found the synthetic molecule—APN01—attracted coronaviruses away from real human airway cells. The virus locked onto the decoy and was marooned there. “We are blocking the door for the virus and, at the same time, protecting tissues,” Penninger says. Apeiron is planning clinical trials later this year for APN01, which must be administered in the hospital as an infusion to sick patients.
EMERGING from a cell, SARS-CoV-2 virus particles (red circles) will create a wider infection unless drugmakers find ways to block them. Credit: NIAID-RML
OVERREACTIONS
In the sickest COVID-19 patients, a mass of mucuslike fluid accumulates in the lungs, preventing cells from absorbing oxygen. These are the patients that need ventilators. The fluid buildup is the result of an overactive immune response that involves a signaling chemical called interleukin-6 (IL-6). Biotech companies, including Regeneron and Genentech, have manufactured synthetic antibodies that can bind to IL-6 and mute the call to action that it sends out.
Northwell Health, a large system of 23 hospitals based in Long Island, N.Y., is one of more than a dozen centers participating in clinical trials of the IL-6 blockers, says Kevin Tracey, chief executive of the Feinstein Institutes for Medical Research, which is running the trials at Northwell sites. “The hospitals are being inundated with very sick patients suffering from serious pneumonia and acute respiratory distress,” Tracey says. “The IL-6 drugs have a plausible mechanism of action. I’m optimistic they’ll work.”
None of these approaches are cures. Denison says the drugs under development may “reduce the severity” of an advanced COVID-19 episode, especially if they can be administered when initial symptoms—a mild cough, muscle aches or slight fever—first arise. In a hopeful future, a combination of various therapies may be able to thwart the virus on several different fronts, the way a cocktail of antivirals can beat back an HIV/AIDS infection. By limiting symptoms, drugs may be able to keep some patients out of the hospital and keep hospitalized patients off of ventilators. They can serve as a bridge to survival as other scientists rush to develop the real virus slayer: a vaccine.
Read more about the coronavirus outbreak from Scientific American here. And read coverage from our international network of magazines here.