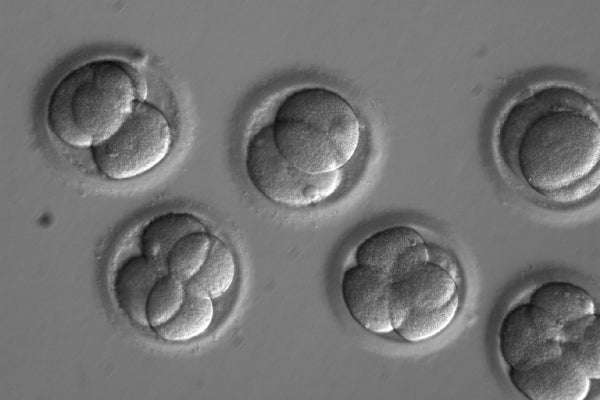
When it comes to CRISPR, questions about if we can edit human embryos are fast giving way to discussions more focused on “But should we?” and “When?” as feats with the gene-editing technology have started to accrue.
Today, biologists from Oregon report in Nature that they have had unprecedented successes using that gene-editing technology to alter early-stage, viable human embryos. The advance moves the field far past earlier attempts by researchers in China and underscores the need to come up with some answers—and fast, researchers say. Although the U.S. Food and Drug Administration is currently barred from granting approval to anyone hoping to use this technology in pregnancies, the Nature study suggests such work could be possible, says Jennifer Doudna of the University of California, Berkeley, a biochemist and CRISPR expert. But she and many others say this would be an inappropriate use of the technology. “I’m not categorically against all human germ-line editing,” Doudna says, “but I think there would need to be a reason to do it that would justify the risks and costs.”
The Oregon Health & Science Universityteam edited the DNA of dozens of embryos to correct for a genetic mutation that often leads to heart failure. They then checked the edited embryos with genome sequencing and discovered that the procedure caused no apparent errors. The group also managed to almost completely eliminate mosaicism—an editing failure in which only some of the desired cells are repaired. “Those are the things everyone was concerned about in earlier embryo work,” says George Church, a CRISPR expert andgeneticist at Harvard Medical School, who was not involved with the work.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The gene-editing success appears to be largely due to one procedural change: The researchers introduced the editing system—the enzyme Cas9 and a guide RNA sequence that helps the editing machinery find its target—at the same time they injected the mutation-laden sperm into a healthy egg in the lab. That allowed for much earlier editing to take place—apparently prior to any cellular replication. That’s a departure from earlier approaches by other research groups in China that had instead introduced the editing components after the egg was fertilized by sperm. “This small change in injection protocol has resulted in something better than all prior embryo work and cell culture work,” Church says.
So far, preventing disease by employing CRISPR–Cas9 to alter the human germ line—a human embryo, egg or sperm—has remained extremely controversial, due to concerns about unwittingly introducing errors or leaving stowaway unedited disease-causing mutations that would put future generations at risk of disease. And until now CRISPR had not been tried on human embryos in the U.S. But Shoukhrat Mitalipov at Oregon and his colleagues went further than the earlier Chinese works, editing dozens of embryos with much greater efficiency.
“This is a harbinger of what’s to come,” says Doudna, who also did not take part in the work. “This underscores the important discussion that needs to happen right now,” she says. George Daley, a stem cell researcher and dean of Harvard Medical School agrees: “This paper establishes that we can do embryo gene editing. The question now remains should we—and for what purposes and should there be certain applications that are allowed and others that are prohibited?”
Such contentious issues were considered earlier this year by a National Academies of Sciences, Engineering and Medicine expert working group. It released guidance saying it would not be appropriate to proceed with any clinical work of human germ-line editing unless there was broad public consensus about the safety and merits of the work—something that has not been achieved. The Academies committee has noted there is still a tremendous amount of research needed before trying to move forward with something like initiating pregnancies. And this Nature paper is just the beginning of the research the report contemplated, says legal scholar and bioethicist R. Alta Charo of the University of Wisconsin Law School, who co-chaired the Academies’ committee but says she is speaking for herself and not the panel. “Understanding how gene editing works in human embryos will require research in human embryos,” because mouse embryos, for example, have species-specific developmental differences, notes Dana Carroll, a biochemistry professor at the University of Utah who researches CRISPR. (The Mitalipov team only allowed the human embryos to proceed to the blastocyst stage—when they are just a few days old—and did not attempt to implant them in a woman.)
In their study Mitalipov and colleagues edited out the MYBPC3 mutation associated with hypertrophic cardiomyopathy (HCM), a disease of the heart muscle that affects about one person in 500. Even a single copy of the mutation can result in the disorder and about 40 percent of individuals with HCM have the mutation. Right now the problematic gene can often be caught with preimplantation genetic diagnosis—screening of embryos in the lab—but using CRISPR could boost the number of usable embryos, they say. Moreover, this CRISPR technique may eventually be an important intervention in situations where parents want to have a genetically related child but have a homozygous condition—say both parents have two copies of a disease-causing mutation like that which causes sickle cell—which would result in all embryos being affected by the disorder.
There are myriad technical obstacles to overcome before CRISPR could address homozygous conditions. One big one: “Not everybody appreciates the way CRISPR technology works—it makes the cut in the DNA but it doesn’t take care of the repair—so we rely on the cells’ fundamental machinery to do that,” Doudna says. In the latest experiments the Mitalipov group focused on snipping out the mutated gene in heterozygous cells—a situation in which there was still a “good” nonmutated copy available for the natural cellular repair systems in the embryo to use as a template for repair after the researchers edited out the problematic one. But in the course of their work, the team noted a potential hiccup for CRISPR work on homozygous forms of the disorder—it may be extremely difficult to repair homozygous mutations once they are edited out because they would not have those built-in blueprints for what they should look like. In fact, the Mitalipov group found that cellular repair mechanisms, at least in their experiments, did not respond very well to the introduction of a synthetic repair template added by the researchers. According to Doudna, that suggests future homozygous work will be challenging—maybe far more so than scientists have expected.