From undulating surface to inky black depths, Earth’s oceans are littered with the carcasses of tiny life-forms called phytoplankton that in life form the basis of the marine food chain.
These microscopic ghosts contain a reservoir of carbon estimated at a staggering 662 gigatons—200 times greater than the amount stored in all living plants and animals—that could come back to haunt us if unleashed from its watery grave as planet-warming carbon dioxide.
Some of the carbon-containing molecules in these plankton remnants will remain locked away for millions of years on the seafloor, but some will break down and enter the atmosphere as carbon dioxide. A huge portion will continue to circulate freely in the ocean for generations. But exactly which molecules are destined for which fate—and therefore how much of this vast carbon pool is headed for the atmosphere via ocean warming, acidification, sunlight or digestion by microbes—is an outstanding question. Answering it requires a clearer picture of the structure of the molecules that contain this carbon. An international team of scientists has now taken the first “photographs” of these molecules in an effort to start parsing that out. This first glimpse suggests that while a catastrophic breakdown and release of carbon seems unlikely, there is much left to understand about the behavior of oceanic carbon.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Only about 10 percent of an estimated 5,000 planktonic carbon-containing molecules have been identified, despite existing in such vast quantities; most structures have only been hypothesized. Getting an actual look at them is challenging because collecting a mere milliliter for study requires filtering 3,600 liters of seawater. And the technique previously used to decipher what atoms were present required busting the molecules apart, giving little insight into the chemical bonds that held them together in the first place.
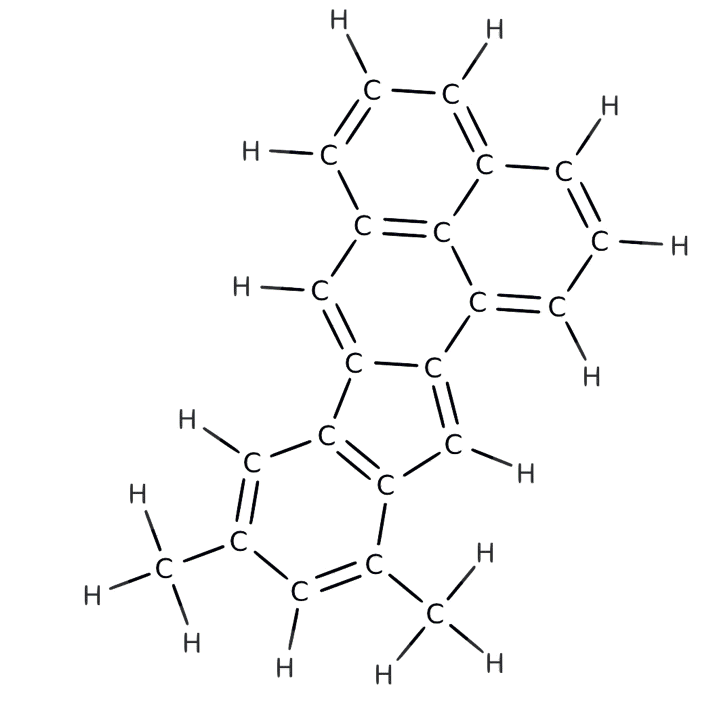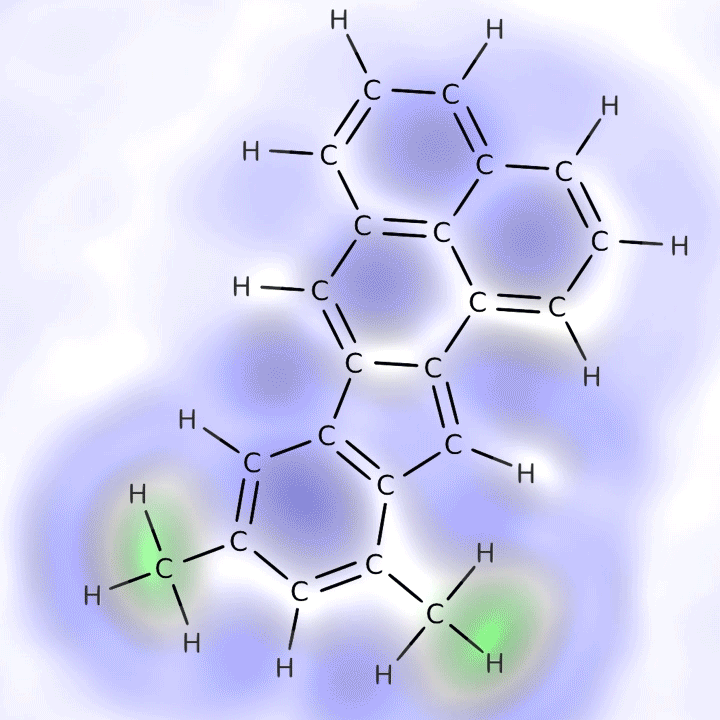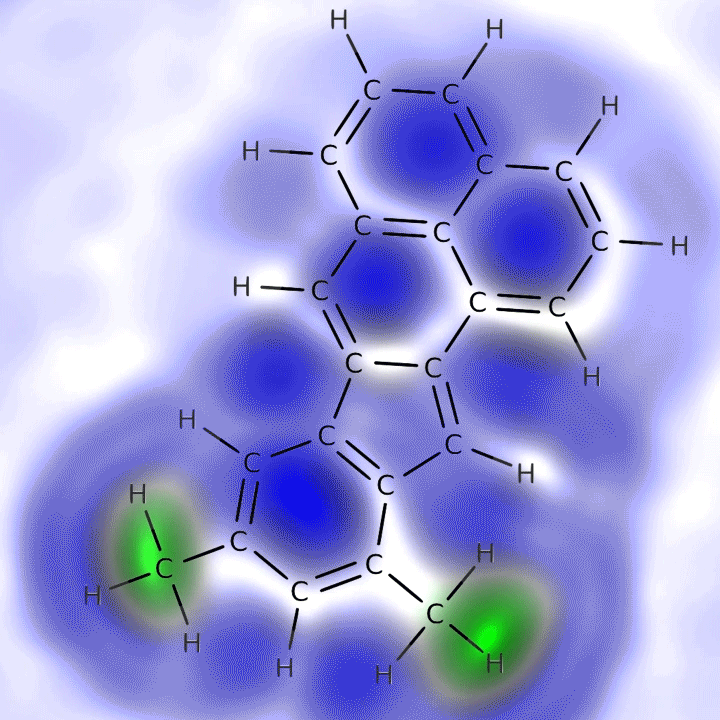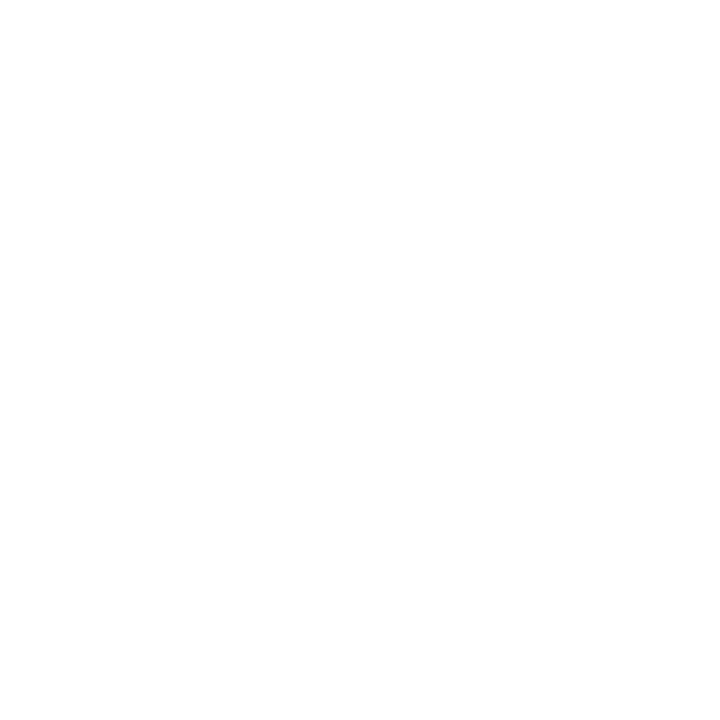
An animation of the molecular skeleton of a carbon-containing molecule from the ocean overlain with its actual chemical structure. This structure is one that appears more resistant to breaking down. Credit: IBM Research
In the new study published this month in Geophysical Research Letters, the team used a technique called atomic force microscopy to create visual images of carbon-containing molecules. First the researchers isolated the molecules atop an incredibly flat surface in a deep vacuum, cooling them to –450.7 degrees Fahrenheit to make them sit very still. Then a minuscule scanning arm with a single carbon monoxide molecule on the tip moved across the subject molecules’ surfaces to measure peaks, valleys and overall shape. (Carbon monoxide has been shown to be the most responsive molecule for measuring these atomic dimensions.) The researchers found molecules from near the ocean’s surface were difficult to image because they were “three-dimensional and bulky,” says team member Leo Gross, an expert in atomic microscopy from IBM Research—Zurich. “The deeper molecules were easier because their surface was planar.”*
The study found the deep molecules are smaller and have a ring structure, which helps them resist forces that might otherwise degrade them; this allows them to accumulate in the ocean. Radiocarbon dating indicates some of them have been circulating through the depths for more than 4,500 years whereas surface molecules may be only months old, because most are quickly gobbled up by microbes or broken down by sunlight or other biological processes. Team member Alysha Coppola, an oceanographer at the University of Zurich, suggests the deep molecules might be too diffuse for microbes to find—or that these remarkably hardy molecules might themselves be by-products of microbe digestion.** “They’re kind of like rubber tires in the ocean” that are likely unappetizing to microbes as well, she says.
University of Miami oceanography professor Dennis Hansell, who has studied dissolved oceanic carbon extensively but was not involved in the new study, says the ability to image actual molecules represents an important advance. “We ocean scientists do not know the protections in place that prevent this material from being consumed by microbes,” he says. “So these visually compelling results move us closer to that understanding.”
The differences in structure and stability between the surface molecules and those found in the ocean depths are just the starting point for understanding whether most of this carbon will stay locked away for the foreseeable future—or if conditions such as warming, acidity or reductions in dissolved oxygen might trigger a much more rapid breakdown. “It has been cycling for millennial time scales—and given its structure, I don’t think it will change,” Coppola says. “But small alterations in climate could make a big difference because there’s just so much carbon—and it’s present everywhere.”
Editor's Notes (8/8/18):
*This paragraph was edited after posting to correct Leo Gross's affiliation and the name of the type of microscopy used.
**This sentence was edited after posting to update Alysha Coppola's affiliation.