The brain relies on a system of chemical messengers, known as neurotransmitters, to carry missives from cell to cell. When all is well, these communications enable the brain to coordinate various functions, from complex thought to quick, knee-jerk reactions—but when the system is out of whack, serious disease or disorder can ensue.
A team of researchers at the Technical University of Denmark (D.T.U.) and University of Oxford have for the first time identified the molecular structure of dopamine beta-hydroxylase (DBH), the enzyme that controls the conversion between dopamine and norepinephrine, two major neurotransmitters. Understanding the crystal structure of the enzyme could provide an ideal target for drug development.
Dopamine and norepinephrine play key roles in many brain functions such as learning, memory, movement and the fight-or-flight response. Imbalances in the levels of these neurotransmitters—and the role DBH plays in regulating them—have been implicated in a wide range of disorders, including hypertension, congestive heart failure, anxiety, depression, post-traumatic stress disorder, Alzheimer’s, schizophrenia, Parkinson’s and even cocaine addiction.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
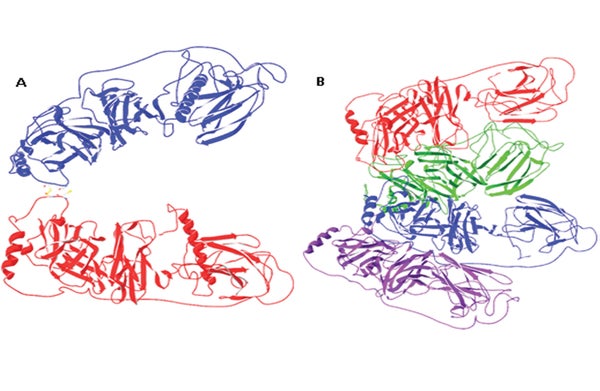
DBH has long intrigued biochemists but it has been challenging to perform the analyses needed to determine the protein’s structure. “This enzyme has been particularly difficult,” says Hans Christensen, a chemist at D.T.U. and the study’s lead researcher. “We tried many different expression systems before we finally succeeded. Now that we have the structure it is clear why—[it] is very intricate, with different parts of the enzyme interacting very tightly.”
Using x-ray crystallography, a technique used to determine protein structure, Christensen and his team found that DBH contains two potential binding sites: first, a pocket where another molecule can bind, common to the class of enzymes to which DBH belongs. There was, however, also a binding site for metal ions that was entirely unanticipated. “What we see in the structure of the enzyme is that it is not at all as people have been expecting,” Christensen says.
In fact, the only part of the structure that was previously known concerned the portion of the enzyme that performs catalytic reactions—and now the researchers have discovered that this region may take a second form, in which two copper-binding sites are located much closer together than predicted. “This might in fact be the active form of the enzyme,” Christensen says. “If that is the case, it [would be] easier to understand how the enzyme actually works, but a lot more research is needed to clarify this.”
Mario Amzel, a biophysicist at Johns Hopkins University who did not participate in this research but has studied the crystal structure of a related enzyme, expressed some doubt about this assumption. “In general, I don’t think that the proximity of the two copper sites will be followed up [in future research],” he says. Even so, Amzel is optimistic about the finding. “The structure is a major accomplishment,” he adds. “The molecule is much larger than the catalytic core. What’s expected is that other parts of the molecule are involved in the regulation or modulation of activity in many ways. How that activity is controlled by these other parts of the molecule is a very important direction where the field will start going.”
Knowing the structure of the enzyme could lead to the development of pharmaceuticals that target the two binding sites the researchers located. “Our next step is to link up with the biotech industry to try to help them develop drugs,” Christensen says. Previous research shows that in most cases, this will mean reducing the activity of DBH, although certain disorders may in fact require that the enzyme do more work. DBH inhibitors are already in clinical development for cocaine dependence, hypertension and PTSD—although these target only the copper-binding sites. The latest findings can facilitate more directed research toward new treatments.