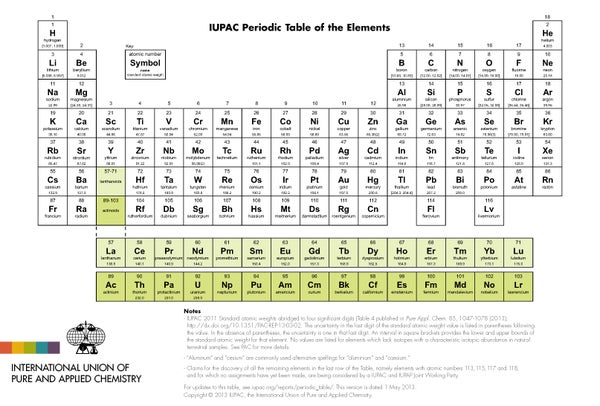
Chemists and physicists have begun 2016 heavy with resolution—superheavy, in fact. Two days before 2015 came to end the guardians of the periodic table of the elements—the International Union of Pure and Applied Chemistry—announced that it was okay to add four new ones, filling out the seventh row. Atoms of each new element are packed with protons in their nuclei, giving the four atomic numbers of 113, 115, 117 and 118.
The official stamp of approval gives the scientists who made these discoveries during the past several years, by slamming lighter nuclei together, the rights to name the elements. The newbies "can be named after a mythological concept, a mineral, a place or country, a property or a scientist," according to the union. Four years ago element 112 was named copernicium, to honor Polish astronomer Nicolaus Copernicus, who placed the sun instead of Earth at the center of the universe.
The permanent names of the new heavy foursome are as yet unknown. Right now they go by placeholders called ununtrium (113), ununpentium (115), ununseptium (117) and ununoctium (118). Their discoverers, who work at the RIKEN Institute in Japan, Joint Institute for Nuclear Research in Russia, and Lawrence Livermore and Oak Ridge national laboratories in the U.S., are doubtless in deep discussion about how their findings should go down in history.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.