The gene-editing method CRISPR has transformed biology, giving scientists the ability to modify genes to treat or prevent genetic diseases by correcting dangerous mutations and to create a host of new genetically modified plants and animals. But the technique, which involves using an enzyme called a nuclease that acts as molecular scissors to “cut” DNA, can cause unintended effects. Making such double-stranded breaks in DNA can result in unwanted genetic material being inserted or deleted, which can have consequences including activating genes that cause cancer. Most mutations cannot be corrected easily without creating these undesirable genetic by-products.
In 2016 a team led by David Liu at the Broad Institute of Harvard University and the Massachusetts Institute of Technology developed another method, called base editing, which allows scientists to make precise edits to single DNA letters without relying on double-stranded breaks. This technique, however, can only be used to fix four out of the 12 types of “point” genetic mutations, which include insertions, deletions and combinations of the two.
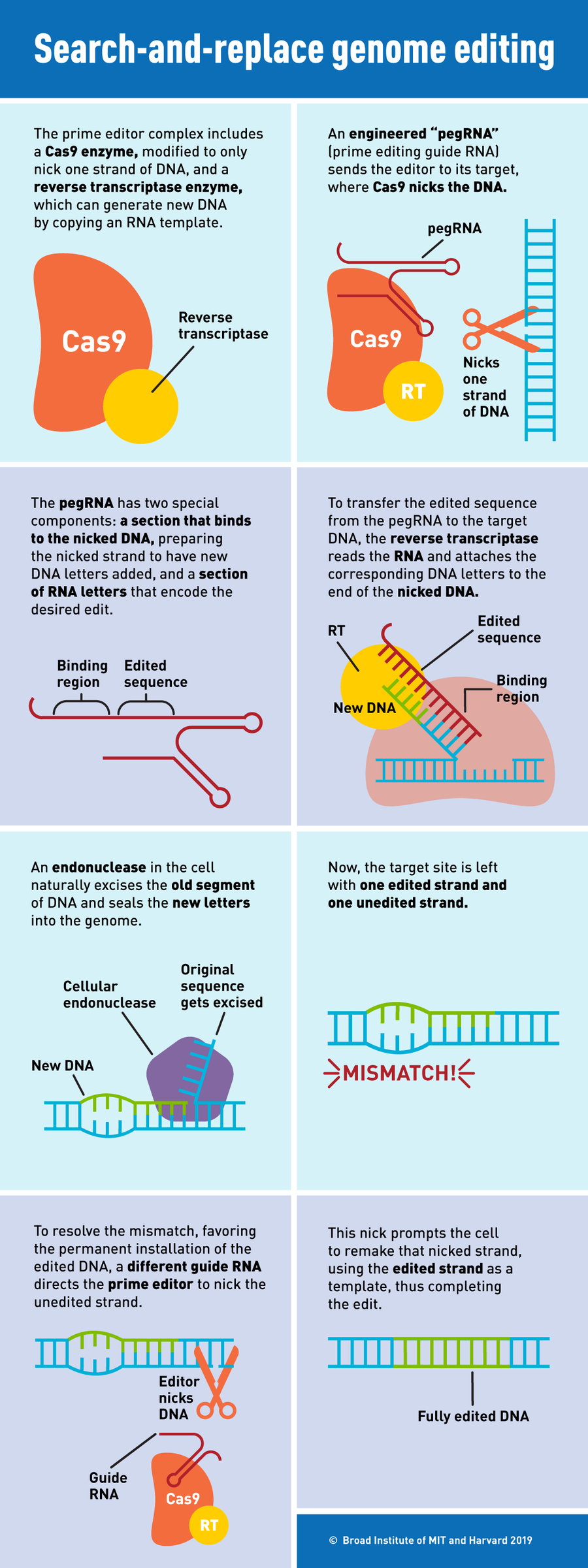
Now Liu, Andrew Anzalone—a postdoctoral researcher in Liu’s laboratory—and their colleagues have developed a new gene-editing tool that avoids these double-stranded breaks and can correct all 12 types of point mutations. The researchers used their new technique, dubbed “prime editing,” in lab-grown human cells to correct the genetic defects that cause sickle cell disease and Tay-Sachs disease, they report in a study published Monday in Nature. They say their method is more efficient than traditional CRISPR (except for in certain cell types) and has fewer off-target effects. In principle, it could correct about 89 percent of known human genetic defects that cause diseases, although it is still a very new technique and requires much more study before it can be used to treat humans.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
“In the big picture, the invention of prime editing is a moment for all gene editors to stand up and cheer,” says Fyodor Urnov, a professor of molecular and cell biology at the University of California, Berkeley, and scientific director for technology and translation at the Innovative Genomics Institute, a partnership between U.C. Berkeley and the University of California, San Francisco. “Prime editing is potentially an exceptionally powerful way to do [gene] repair,” says Urnov, who was not involved in the study but was a reviewer on the paper. Yet he cautions that “in practical terms, these are very early days.”
Credit: S. Hamilton & K. Zusi Broad Institute of M.I.T. and Harvard
Prime editors consist of two components, a protein and an RNA molecule. The first is an engineered form of the common CRISPR enzyme Cas9 combined with a second enzyme called a reverse transcriptase. The second is an engineered guide RNA, called a pegRNA, which both specifies the target site in the DNA and serves as a template for the desired edit. At the target site, the engineered Cas9 makes a nick in one strand of DNA, and the reverse transcriptase directly copies the pegRNA into a new DNA strand attached at that point, letter by letter. This creates an extra “flap” of DNA with the edited sequence. The prime editor then cuts the unedited strand and replaces it with the edited one.
“If nucleases [such as Cas9] are like scissors, and base editors are like pencils, prime editors are like word processors,” Liu says. They serve a kind of “search and replace” function for DNA.
Anzalone and his colleagues compared prime editing with CRISPR’s usual DNA repair mechanism, finding that the new method was more efficient (meaning it successfully edited a higher proportion of cells) and that it produced far fewer insertions and deletions. With prime editing, the team was able to achieve an efficiency of about 20 to 50 percent—and in some cases, as high as 78 percent, depending on the cell type. For certain diseases, such as sickle cell, even 25 to 30 percent efficiency could alleviate some of the symptoms. Others, such as cystic fibrosis, will require much greater efficiency.
The CRISPR enzyme Cas9 is known to have unintended, or off-target, effects at a number of sites in the genome. The researchers showed that prime editing causes fewer off-target effects at these locations. They think this occurs because whereas Cas9 requires only one instance of DNA pairing, prime editing requires three, which means there is a lower likelihood of random pairings that could have unwanted effects.
Other researchers praised the new approach, although they noted that the technique is still in its early stages. Feng Zhang, one of several CRISPR pioneers, who is also at the Broad Institute but was not involved in the study, calls it “a creative approach further expanding the range of genetic changes that can be made.” (Zhang is a co-founder of the companies Editas Medicine and Beam Therapeutics, which are developing CRISPR-based therapies.)
Jussi Taipale, a professor of biochemistry at Cambridge University, calls it a “major advance” that “moves from a genome cutting tool toward a true genome-editing tool.”
“It’s exciting to see another tool in the CRISPR-Cas9 toolbox!” adds Jennifer Doudna, a CRISPR pioneer at U.C. Berkeley, who was not a co-author on the study but collaborates with Liu's lab. (Doudna is a co-founder of Caribou Biosciences, Editas Medicine, Scribe Therapeutics and Mammoth Biosciences—all companies working on CRISPR technology.)
Like every new technique, prime editing has its limitations, which will have to be overcome before scientists can contemplate using it in humans. For one, researchers have to figure out how to deliver the tool to cells in the body in a way that reaches the intended cells safely and effectively. Anzalone and his colleagues explored both viral and nonviral delivery methods in their study, and they plan to do additional studies in animals.
Another issue is whether the engineered enzyme and RNA could activate the body’s immune system, Urnov says. The RNA is originally from bacteria and the enzyme is from a virus, so the two are “a chimaera the likes of which we’ve never seen,” he adds. (Classical CRISPR faces similar challenges, too.)
The introduction of a reverse transcriptase enzyme could also copy other RNA in the cell into DNA that could then be incorporated into the genome, causing unwanted effects, Zhang says.
Finally, there is the efficiency. Zhang says the results they achieved are promising but will need to be improved in certain disease-specific cell types.
Prime editing is not likely to replace techniques such as CRISPR or base editing. “Nuclease editors [such as Cas9], base editors and prime editors all have strengths and weaknesses,” Liu notes. “We anticipate all three classes will have roles in therapeutics and research,” he says.
Urnov likens the different gene-editing tools to dog breeds—each have their own specialized functions. For example, border collies are great at herding sheep, whereas German shepherds make good police dogs. When it comes to gene editing, he says, “I honestly think we do not have enough data on what will be most widely used ‘dog.’”
