On a recent spring day William Cohn, a surgeon at the Texas Heart Institute, waited for a call he hoped would not come. In an operating room near his office doctors worked to extract wires that had been threaded through a 55-year-old female patient’s blood vessels from her pacemaker to her heart. The wires had broken, as happens all too often with pacemaker leads, and now they had to be removed without damaging the patient’s blood vessels. Cohn was on call in case damage did occur and had to be patched in a hurry.
Cardiac resynchronization therapy (CRT) devices, such as pacemakers, that are designed to electrically stimulate the heart to function normally fail to help as many as a third of patients who receive them each year, according to some studies. About 600,000 patients receive pacemakers worldwide every year. The leads connecting the devices to electrodes on or in the heart are a major cause of that failure and, as Cohn can attest from first-hand experience, they can lead to other problems as well. Leads may fracture, break or become dislodged, necessitating additional procedures for replacement.

Image Courtesy of EBR Systems
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
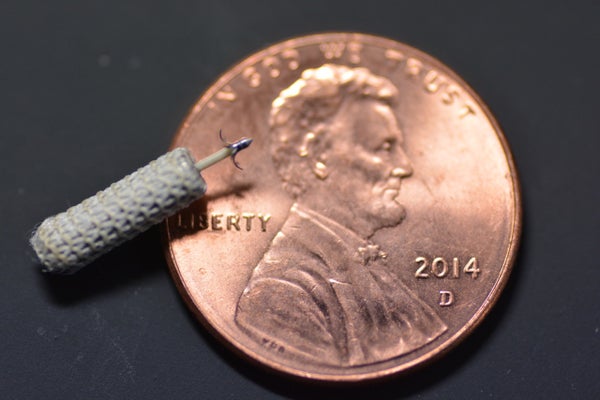
But a new breed of leadless CRT devices have begun making their way into the market, and to doctors like Cohn they are the future. One such device is from a Silicon Valley start-up called EBR Systems. Its WiSE device features a leadless electrode the size of a grain of rice that attaches to the inside of the heart’s left ventricle via catheter. Once there, its piezoelectric materials generate a heart-stimulating electrical pulse in response to ultrasound energy beamed to it by another implant in the left chest cavity. “We’ve done [this in] 53 patients and we’ve had great results,” says EBR CEO Allan Will. “We’ve taken the failures from conventional cardiac resynchronization therapy and we’re fixing over 80 percent of them.”
The three major CRT device-makers—Medtronic, Saint Jude Medical and Boston Scientific Corp.—are also working on leadless technology but with a different approach. They are doing away with electrodes entirely and are instead shrinking their devices to a small-enough size to be entirely implanted—processor, battery and all—in the heart. But at roughly the size of an AAA battery, the devices are still too big to be placed in the left ventricle (where they could form clots that would be more dangerous than those forming in the right ventricle), making them complementary to WiSE rather than directly competitive. In fact, WiSE is designed to synchronize with a conventional pacemaker installed in the right ventricle.
The devices from EBR, Medtronic and Saint Jude have already been cleared for use in Europe, with U.S. Food and Drug Administration clearance pending further clinical trials in the U.S.*
Fortunately, Cohn’s potential patient came through her lead extraction without incident. But Cohn looks forward to the day when he and his colleagues will not have to be on call for such procedures. “The idea of changing the way devices communicate without leads is a superhot topic in medicine,” he says.
*Update: On April 6 Medtronic announced that its Micra Transcatheter Pacing System had become the first leadless pacemaker to receive U.S. Food and Drug Administration approval.