To see what University of York materials scientist Roland Kröger and his colleagues recently saw in their laboratory, you would have to take an incredibly close look at a human bone—closer in than at the scale of its spongy pattern of collagen threads that reportedly inspired the Eiffel Tower’s crisscrossing struts, and the star-shaped cells that populate our skeletons’ pale terrain. You would have to perform painstakingly difficult techniques with a state-of-the-art electron microscope that can resolve details less than 10 nanometers in size—smaller than 10 carbon atoms lined up side by side, or 3,000 times thinner than a sharpened pencil line.
At this level of magnification, the researchers were able to discern novel details about the building blocks of our bodily framework: our bones, which comprise mineral crystallites and spiraling strands of the protein collagen called fibrils. The scientists saw mineral crystals just five nanometers in diameter unexpectedly twisting into and around these collagen strands—forming a helical structure. This offers a new understanding of how our bones’ components are fundamentally organized at the smallest scale, says Elizabeth Boatman, an assistant professor of engineering at Wake Forest University who studies bones and was not involved with the new research. The revelations may help explain bone’s remarkable strength and toughness, which has long been a mystery.
But this discovery, published Thursday in Science, remains mired in a decades-old debate about the complexities of bone at the nanolevel. Since the first electron microscopes turned their beams on bone in the late 1960s, scientists knew it was primarily made up of bone mineral and collagen, a fibrous protein. But they have since argued about how these two constituents are organized relative to each other. Some researchers thought the mineral was mostly contained inside fibrils; others believed the mineral cladded the protein like armor.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
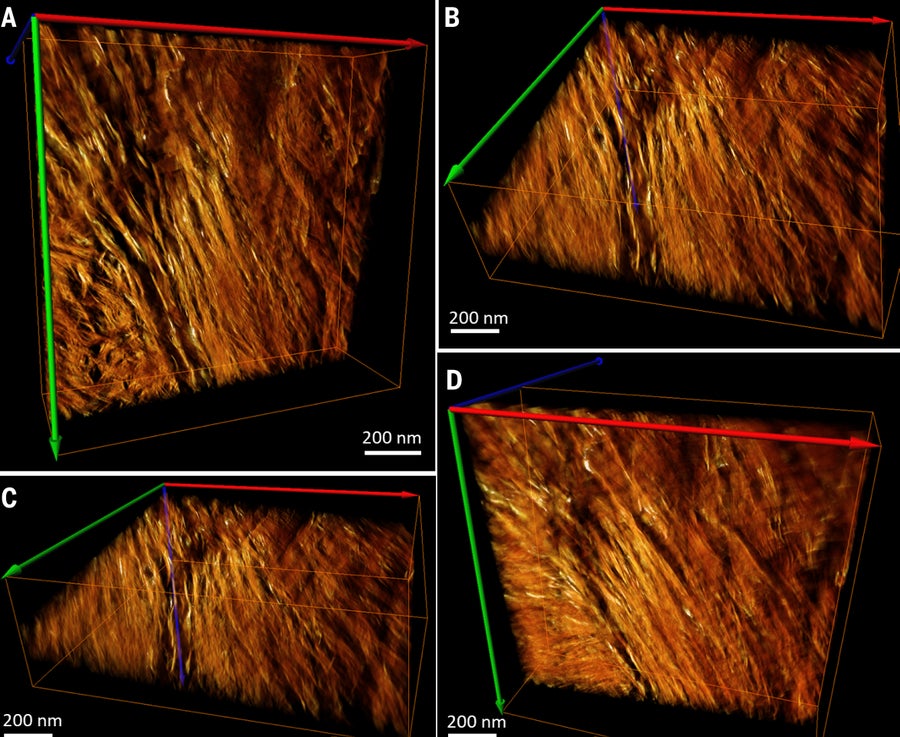
Reconstructed and rendered bone image. Credit: N. Reznikov et al., Science 2018
Kröger and his team believe they may have solved this mystery by using state-of-the-art electron microscopes, which create images by interpreting how a beam of electrons scatters when passing through a sample. The researchers also prepared their bone samples in an unconventional way aimed at keeping intricate nanostructures intact. “Most previous papers use a technique for cutting bone, which results in the material in the bone being totally screwed up,” says Henry Schwarcz, a geochemist studying human bone structure at McMaster University who did not work on the new study. In his own research he has demonstrated that the predominant method, which uses a conventional bladed instrument for cutting, can shatter nanoscale bone features as it slices.
For this study the researchers instead used a focused ion beam to slice bone into samples for microscope analysis. This, Schwarcz says, can mill out excruciatingly small and thin sections of bone without obliterating its atomic-size structures. The team managed to capture three detailed pictures of these carefully sliced, mineralized collagen fibrils—protein–mineral composite threads that make up most of our bones. Two of the images showed a pattern already familiar to nanoengineers and materials scientists working with bone: a stringy, filamentous arrangement Schwarcz had previously hypothesized to be a profile view—a lengthwise shot of bone—and a lacy, webbed pattern thought to be a cross-section of fibrils.
The third image, however, revealed a pattern nobody had ever reported seeing: Dark gray lines swirling into the center of closely packed hexagons with smaller, black hexagonal dots studded across them. Kröger, the senior author on the paper, calls them “rosettes.” At first he thought he was seeing just another cross-section of the tiny mineralized collagen fibrils, possibly in an orientation that had never been sampled. “Every time you see something that is unexpected, it is quite exciting and puzzling at the same time,” he says. “We thought, ‘What’s happening here?’ This spiral arrangement we hadn’t seen before.”
The team took electron microscope pictures of one of their ultrathin bone sections from different angles, rotating the sample by 1 degree each time. Then they fed all of the images into a computer that reconstructed a 3-D model, called a tomogram, of the sample.
This new model showed needle-shaped bone mineral crystals twisting into and around the collagen fibers, entangling them in a stiff, spiral scaffold. The resulting shape resembles a lot of fraying, hardened fibers woven into a flexible rope. “We saw many of those crystals, splaying out in variations of about 20 degrees in different directions. That shows the projection of these twisted nanocrystals going in and out of the collagen and entering neighboring collagen fibrils,” Kröger says. The result is “an intricate network, or mesh, of the nanocrystals.”
But other researchers have a few bones to pick with this model. In his research, Schwarcz says, he has never seen anything suggesting bone minerals are structured into curved, needle-shaped crystals. A body of literature based on a different imaging technique, called small-angle x-ray scattering, indicates bone nanocrystals are plate-shaped, he says. “I’m willing to reserve judgment because there may be something I’m missing here,” he adds. “But I’m really hard-pressed to understand how this can be.”
How or why these crystallite needles are helically organized remains an unanswered question. During the sample preparation, collagen fibrils contract from dehydration—and this may have stretched the crystals into a curved shape when they might have been straight in the body, Boatman speculates. “That’s my big concern,” she says.
But if the model turns out to be correct, it might help explain why our bones are so remarkably strong. “The helical nature—that’s novel,” says Iwona Jasiuk, a mechanical and bioengineer at the University of Illinois at Urbana–Champaign who did not participate in the work. Like a coiled spring, a helical structure can support greater loads before breaking compared with simple linear structures, she notes. “[The structure] should enhance bone toughness and strength.”
That principle could one day be incorporated into manufacturing processes, Jasiuk adds. Inspired by the bone model Kröger and his colleagues propose, developers could begin designing a new generation of stronger, more durable, lightweight building materials reinforced by a helical skeleton within.