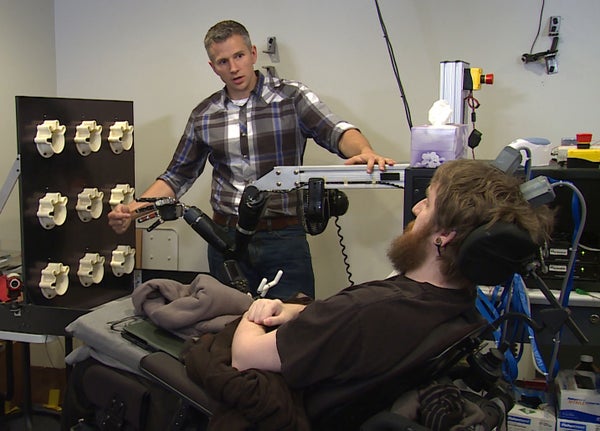
True neuroprosthetic limbs—artificial limbs that feel and behave like the real thing—may be in the distant future. But the results of a new study have brought the technology one step closer to reality. A team led by researchers at the University of Pittsburgh has electrically stimulated the brain of a paralyzed man, allowing him to feel the sensation of touch in his hand again. Recreating that sense fully is one of the greatest challenges facing the field of neuroprosthetics.
“For most tasks that involve manipulation of objects, you’re really relying on the sense of touch to guide movement—you’re not using vision, necessarily,” says Jennifer Collinger, an assistant professor of physical medicine and rehabilitation at the University of Pittsburgh and a coauthor of the study, published today in Science Translational Medicine. “You don’t have any visual feedback on how hard you’re squeezing it, or what you need to do to maintain stable posture. All of that comes from the sense of touch.”
In the study, surgeons implanted electrode arrays in the brain of a 28-year-old man who was paralyzed from the neck down due to a spinal cord injury sustained 10 years earlier, specifically targeting the somatosensory cortex, the brain area responsible for the sensation of touch. Prior to surgery, the researchers used magnetic resonance imaging (MRI) and magnetoencephalography (MEG) to determine where to place the electrodes by noting which areas in the somatosensory cortex were activated when the study participant imagined sensation in different parts of his right hand. Once the electrodes were implanted, the researchers delivered mild electrical currents to the patient’s brain, a technique known as intracortical microstimulation.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
During an initial four-week period in which the participant did not detect any electrical stimuli, the participant experienced spontaneous feelings of tingling in his right arm and hand. These spontaneous sensations often co-occurred with the firing of neurons in the somatosensory cortex. After three weeks, the patient experienced these spontaneous sensations only in his right hand; and after four weeks, the patient stopped perceiving them altogether. Around the same time, the intracortical microstimulation began to produce tactile sensations that the participant reported originating in his hand.*
Collinger and her colleagues conducted two different types of trials, both involving intracortical microstimulation of the somatosensory cortex. In the first, the participant received stimulation while viewing a diagram of a hand on a screen. He was asked both to describe the sensations he experienced and to locate where in his hand he felt them. The participant reported primarily experiencing sensations in his upper palm and at the base of his four fingers, with sensation in a small area of his pinky as well as two-thirds of the way up his index finger as well.
In 93 percent of trials intended to measure the naturalness of the experienced touch, the participant described the feelings as “possibly natural,” with none feeling “totally natural” or “totally unnatural.” In 94 percent of trials measuring the depth of sensation, the participant reported experiencing the feeling as occurring both on and beneath the skin. In trials meant to study the perception of pain, none elicited a painful response from the participant. And in 190 trials meant to determine the quality of the experienced sensations, he reported 67 percent of stimuli registering as pressure, 15 percent registering as warmth and another 15 percent as electrical stimulation. In one of these trials, the participant felt the simulation as an unpleasant vibration.
In the second part of the experiment, the researchers mapped the output of torque sensors to specific fingers on a prosthetic hand, which were in turn mapped to the electrodes corresponding to each finger. The participant was then blindfolded and asked to report in which finger he felt pressure being applied. Across 13 sessions, in which 62 to 65 trials apiece were conducted on four fingers, the participant correctly identified which finger was being stimulated 84 percent of the time.
All of these responses remained stable over the course of the six-month study. “We were hopeful that these would be the results, but it’s definitely been surprising how consistent these responses are over time,” Collinger says.
This study answers a long-standing question in the field about what stimulating the somatosensory cortex feels like, says Lee Miller, president of the Society for the Neural Control of Movement, who was not involved with the study. “Those of us doing this kind of work in monkeys often preface our presentations by saying, ‘Boy, it certainly would be nice if we could ask the monkeys what it feels like,’” Miller says. “The fact that the patient described the sensations he felt as ‘possibly natural’ gives insight into the direction this work has to go, even as we recognize how far we’ve come.”
Collinger and Miller agreed that the next step is figuring out how to combine the existing discoveries in this field building on previous experiments showing that monkeys and humans can learn to feel virtual objects and control a robot arm with their minds.
Collinger and Miller agree that the next step is figuring out how to combine the existing discoveries in this field. Prior work has shown that monkeys can be trained to feel virtual objects and that both monkeys and humans can operate a mechanical arm using their thoughts. To that body of knowledge, the present study adds data about how people who have lost the use of their limbs might learn to feel again.
“The next immediate step is to integrate motor control and sensory feedback,” Collinger says.
*Editor’s Note: (10/13/16): This paragraph was modified to clarify the sensations (both spontaneous and electrically elicited) the participant experienced during the study.