Sebastian Tobler’s doctors thought his legs would never move again after a mountain-biking accident completely paralyzed them. When he signed up for a study at Lausanne University Hospital in Switzerland, Tobler’s injury was so severe that the researchers had doubts their experimental treatment would work.
“I was like, should we enroll this participant? It’s very rare to see a spinal cord that was this silent,” says Grégoire Courtine, a neuroscientist at the Swiss Federal Institute of Technology, referring to an almost undetectable level of electrical activity in Tobler’s lower spine.
But Courtine and his team have been working on a new technology intended to treat spinal cord injuries like Tobler’s. It involves an electrical stimulator surgically implanted at the base of the spine, under the bones but over the spinal cord. A couple of weeks after the device went into Tobler’s back, Courtine tested it by asking Tobler to try walking.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
To support his weight and allow him to stand, Tobler was strapped into a harness that hung from the ceiling. When the implant was turned on it sent a series of rhythmic shocks into his spine, like an electrical drumbeat. For two hours Courtine tuned the frequency and strength of the shocks while Tobler strained to walk. It had not taken this long to see results for Courtine’s other study participants, whose injuries were not as severe. But then Tobler began taking sudden, jagged steps on the treadmill.
“Oh my god. I’m walking like a horse,” Tobler said.
“Unbelievable!” Courtine’s postdocs said.
Tobler is one of three patients in this study, published Wednesday in Nature, who regained movement in their lower limbs with the aid of implanted electrical stimulators. It is the third study of its kind, the previous two conducted by researchers at the University of Louisville and the Mayo Clinic. But unlike previous trials, two of Courtine’s patients were able to stand and walk with crutches outside of the clinic without the implant activated.
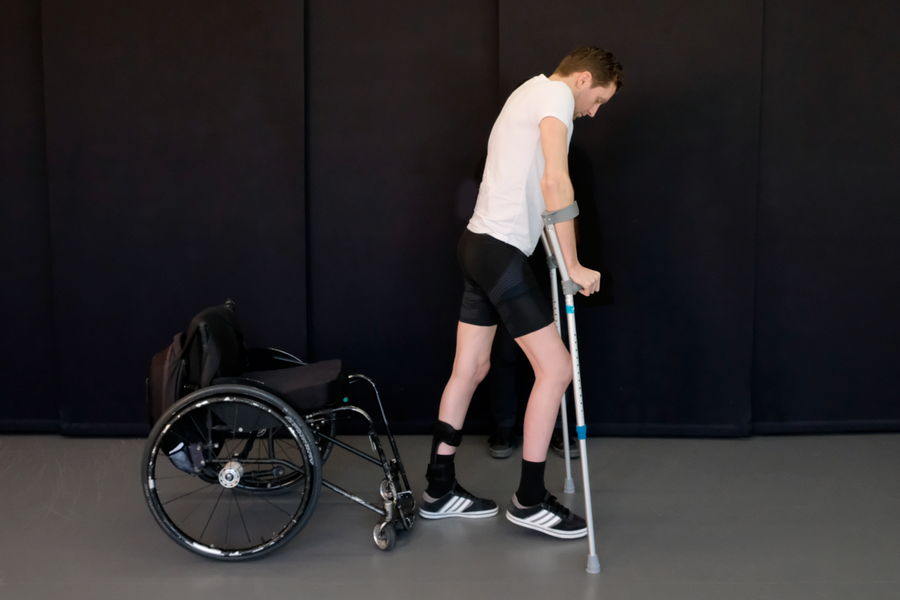
Gert-Jan Oskam, one of several patients in the study. Credit: Jean-Baptiste Mignardot
In most spinal cord injuries the spine is not severed, but bruised. Such damage strips nerve fibers of the lining that helps them efficiently carry electrical signals, similar to the way a rubber sheath insulates a wire. When a command from the brain hits a spinal cord injury, that electrical signal dissipates and becomes too weak to effectively activate muscles.
Scientists think the implant’s electrical stimulation adds enough power back to the brain’s commands to get muscles moving again. “That’s one of the most exciting parts of this,” says Chet Moritz, a neuroscientist at the University of Washington who was not involved with the new work. “It’s enhancing the brain’s ability to control the legs. We think it acts as an [electrical] amplifier for the spinal cord.”
Whereas other researchers’ implants have provided continuous electrical stimulation, Courtine’s version fired electrical pulses in rhythmic patterns that mimic the brain and spinal cord’s natural signaling. Moritz says this staccato action might be why Courtine’s patients reportedly showed stronger signs of recovery than those in previous studies did. “By delivering the patterned stimulation, they were able to be more coordinated and fluid in stepping,” Moritz says. When Courtine had the implant fire continuously in the same patients, that coordination went away.
Continuous electrical stimulation of the spine may overwhelm communication the lower limbs are trying to send to the spinal cord, Courtine and his colleagues report in a second paper also published Wednesday in Nature Neuroscience. “Stimulators cause the nerves to fire in both directions: a natural direction but also backwards, like going down the wrong way on a one-way street,” Moritz says. Any natural information coming up from the leg into the spine—like physical sensations and position in space—might collide with the stimulator’s artificial signal, causing them to cancel each other out.
That in turn makes it difficult for the spinal cord to coordinate movement, says Jocelyne Bloch, a neurosurgeon at Lausanne University Hospital who worked on the new studies. “The collision of signals is confusing the brain and the patient,” she says. But if the implant produces electrical stimulation in pulses, enough natural information might be able to squeeze through the gaps in the rhythm to help patients coordinate their movements better.
After a few months of treatment all three participants were able to move their limbs without the implant turned on. Thus, it seems the months of intense physical therapy and use of the implanted stimulator are actually causing the spinal cord to heal—but what that apparent healing looks like is up for debate. Courtine thinks the spine might be regenerating nerve fibers that cross the injury like new lanes being built around a broken highway section. Or, as Moritz believes may be the case, damaged neurons that were already crossing the injury are rebuilding their lost insulation and simply beginning to work better.
All the participants continued to improve during the five-month course of the study, Courtine says. “Toward the end is when they were improving the best,” he says. None of them can walk efficiently enough to outpace a wheelchair, but he says they are walking nonetheless. “Two of them can walk long distances with crutches,” he adds. “And [Tobler] needs a walker.” Courtine built a mobile app that allows the patients to turn their stimulators on or off remotely, so they could train at home after the study ended. He is also starting a new company called GTX Medical to continue helping patients after the study, and to promote the technology.
Something else also happened to the patients during the study, Bloch says. After a spinal cord injury many people lose other bodily functions such as sweating, bladder and bowel control, and sexual function. To some patients, regaining these functions is more important than walking. “One patient started sweating much more,” Bloch says. And others began having more sensation in parts of their upper bodies. “The healing has not only been for walking,” she says.
In any case, being able to stand and walk a little has been a symbolic victory for the study participants. Gert-Jan Oskam could only make slight movements with his legs before the trial, but now he says he can stand to do simple tasks at home—including cooking and grilling. “Someone has to hold me for security reasons, although I should be able to have a barbecue standing on my own in the near future,” he said in a written statement. “The clinical trial has given me hope.”
