If you were dropped into virtually any region of North America 56 million years ago, you probably would not recognize where you had landed. Back then, at the dawn of the Eocene epoch, the earth was warmer and wetter than it is today. A sea had just closed up in the middle of the Great Plains, and the Rocky Mountains had not yet attained their full height. The continent's plant and animal communities were dramatically different. In the Canadian High Arctic, which today harbors relatively few tundra plant species, year-round temperatures above freezing nurtured a rich and diverse flora; Ellesmere Island in far northern Canada, across from the northwestern coast of Greenland, was home to alligators and giant tortoises. What is now the southeastern U.S. was dominated by tropical rain forest, complete with primates. The northeastern U.S., for its part, ranged from broad-leaved (as opposed to needle-leaved) evergreen forest to deciduous forests of ginkgo, viburnum, birch and elm, among other species. The deciduous broad-leaved forests that now cover 11 percent of North America north of Mexico were in their infancy. But that was about to change, with the spread and extraordinary diversification of what would eventually become some of the most ecologically and economically significant woody plants in the world: the acorn-bearing, wind-pollinated trees we call oaks.
Over the course of some 56 million years, oaks, which all belong to the genus Quercus, evolved from a single undifferentiated population into the roughly 435 species found today on five continents, ranging from Canada to Colombia and from Norway to Borneo. Oaks are keystone species, foundational to the functioning of the forests they form across the Northern Hemisphere. They foster diversity of organisms across the tree of life, from fungi to wasps, birds and mammals. They help clean the air, sequestering carbon dioxide and absorbing atmospheric pollutants. And they have shaped human culture, feeding us with their acorns and providing wood to build our homes, furniture and ships. Indeed, oaks have proved so valuable to people that we have immortalized them in legends and myths for centuries.
Oaks are especially prominent in the Americas. Approximately 60 percent of all Quercus species live here. This astounding variety, along with the fact that the oaks in this region account for more forest tree biomass than any other woody plant genus in North America and Mexico, makes them the single most important group of trees in the continent's forests. To understand forests, then—their biodiversity, food webs and contributions to human well-being—one must understand how oaks came to rule them. For decades scientists could only speculate about much of the evolutionary history of oaks because of gaps in their fossil record and limitations of the biomolecular techniques used to infer evolutionary events from the DNA of living organisms. But recent advances in genome sequencing and analysis have allowed us and our colleagues to reconstruct a detailed picture of the origin, diversification and dispersal of oaks. It is a remarkable evolutionary success story, one that will have important implications for predicting how these essential trees will fare in the face of climate change—and for developing management plans to ensure their survival.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Red and White
The differences between major groups of oaks are readily apparent to even a casual observer. In the Americas, oaks are dominated by two evolutionary lineages that you may already know. One of these, the red oak group, is composed of species with bristle-tipped leaves. In most red oak group species, pollen takes a full year from the time it lands on the female flower to fertilize the seed, so that acorns—the fruits of these trees—pollinated in one year only ripen in the next. Species in the other major lineage, the white oak group, have no bristles on their leaves, and the leaves generally contain more soil-enriching nutrients when they fall than those of red oaks do. Also, white oak acorns almost all ripen the same year they are pollinated, sometimes germinating before they even fall. Gray squirrels preferentially cache red oak acorns to eat at a later date because they are less likely than white oak acorns to go bad before the squirrels can get back to them.
White oaks are also able to efficiently plug the water-conducting, tubelike cells called vessels in their wood with tyloses, balloonlike structures that seal the vessels as a barrier against deadly fungal diseases such as oak wilt. Red oaks are slower and sloppy in their defense. Consequently, white oaks have long served as wood for ships and wine barrels because the plugged vessels of the white oak species hold water more effectively than those of the red oaks. Chewing insects recognize the differences between red and white oaks, and most are adapted to favor either one or the other of these two groups. Even mycorrhizal fungi, which connect plant roots to soil nutrients, appear to recognize the differences between the two types of oaks: many favor symbiotic relationships with species in one lineage over the other.
When we get to the species level, however, closely related oaks are often difficult to tell apart. The variation within species, the result of both plastic responses of the trees to their environment and genetic variation between individuals, often appears to be as great as the variation between species. And oaks hybridize commonly within their group, be it the white or red lineages or any of the six other major lineages of oaks worldwide. These two factors—high variation within species and ongoing hybridization between species—complicate classification.
Hybridization can also make it difficult to reconstruct the evolutionary history of oaks using traditional biomolecular techniques, which involve sequencing one or a few genes, because individual genes often trace different histories. Moreover, a single oak species may have hybridized with numerous different species, so that different genes record different aspects of this history across the geographical range of the species. The oak genome is thus a mosaic shaped by speciation and hybridization. The sequences of only one or a few genes cannot reveal the full history of speciation in oaks.
Two decades ago researchers had only the sequences of DNA from chloroplasts—the cell organelles that carry out photosynthesis—and a few nuclear genes to go on. It was enough to discern the overall branching structure of the oak tree of life, but we could not see the arrangement of its endmost branches. In 2008 the three of us realized that new molecular techniques we were already using to study hybridization and the limits of species in the red oak group might also enable us to infer oak evolutionary history. Since then, we, in collaboration with colleagues around the world, have employed an approach called restriction-site associated DNA sequencing to read short regions of DNA from across the genome. We analyze these data using statistical methods that reconstruct the order in which species have branched from common ancestors and which ones have hybridized since that divergence. By marrying these analyses to fossil data, we can estimate the maximum ages of key events in oak evolutionary history. Despite the complex genetic history of oaks, we have been able to deduce much of the history of speciation in this group going back to the root of the oak tree of life.

Red oaks have bristle-tipped leaves (top); the leaves of white oaks lack bristles (bottom). Credit: John Seiler Getty Images (top); Getty Images (bottom)
Southward Bound
We may never know precisely when or where the very first oaks arose, but roughly 56 million years ago a population of oaks growing near what is now Salzburg, Austria, left in the mud a bit of the massive amount of pollen they produced each spring. These pollen grains, which are shaped like a rugby ball with three grooves running lengthwise and with surface textures that vary by lineage, are the earliest unambiguous fossil evidence of oaks on record. Throughout the early Eocene, land bridges spanning the Atlantic and Pacific Oceans connected North America and Eurasia. Plants and animals freely crossed between the two continents. Oaks were most likely part of a vast forest that spread across the continents of North America, Europe and Asia. This makes it difficult to say with any confidence whether oaks originated in Eurasia and sent a branch off to the Americas, or vice versa. The better answer to where modern oaks arose may simply be “in the north.”
In any case, remarkably soon after they arose, oaks started to separate into two major branches: one limited to Europe, Asia and North Africa and the other largely limited to the Americas. The separation between continents was imperfect at first. For example, the oldest fossil attributable to the ring-cupped oaks, based on the concentric rings formed by the woody scales on its acorn cap, was deposited in Oregon around 48 million years ago. Today this lineage is restricted to Southeast Asia. And red oaks, which today are an American group, have been reported from fossil sites in Europe dating to some 35 million years ago. But when global temperatures started their long descent about 52 million years ago, oaks were gradually pushed southward, away from the land bridges that have connected Eurasia and North America intermittently over the past 50 million years. As cooling drove northern oak populations extinct, the divisions between the two continents became very clean, with no species from the Eurasian clade showing up in the Americas and only two branches of the American clade showing up in Eurasia.
Before they could be pushed too far to the south, oaks were further subdivided into the eight major lineages we recognize in modern forests. Three of them are restricted to the Americas: the red, golden cup and southern live oaks. One lineage, that of the white oaks, originated and diversified in the Americas but sent an offshoot back to Eurasia. We know these major lineages arose early in oak evolution because one of the oldest American oak fossils is a 45-million-year-old white oak from Axel Heiberg Island in Nunavut, Canada, that can be distinguished from the red oaks and all other major lineages of oaks. But fossils from this initial phase of diversification are hard to assign to any one lineage, so we rely on molecular data to estimate when the other oaks separated into independent lineages. The integration of molecular data with selected fossils indicates that the world's eight lineages split early on. It is an important part of the story because it explains what happened next as the North American oaks underwent their own burst of diversification.
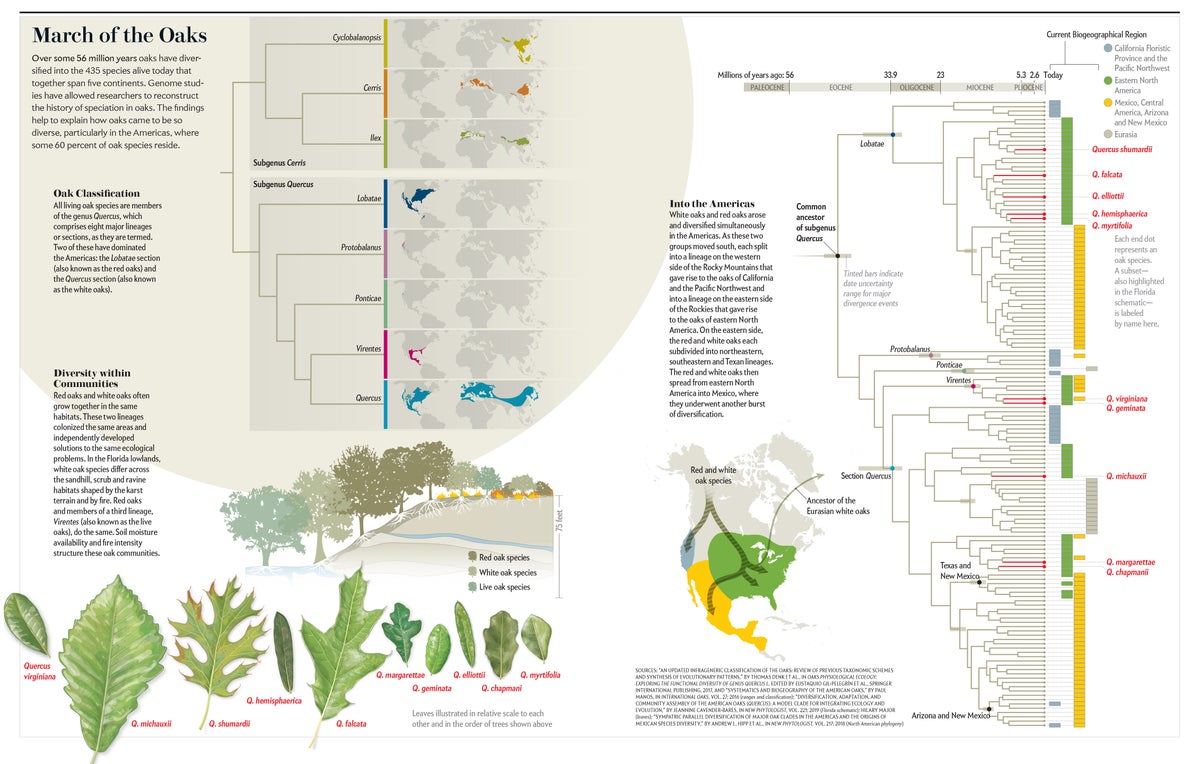
Credit: Daisy Chung (illustration), Mapping Specialists (maps); Sources: “An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Patterns,” by Thomas Denk et al., in Oaks Physiological Ecology: Exploring the Functional Diversity of Genus Quercus L. Edited by Eustaquio Gil-Pelegrín et al., Springer International Publishing, 2017, and “Systematics and Biogeography of the American Oaks,” by Paul Manos, in International Oaks, Vol. 27; 2016 (ranges and classification); “Diversification, Adaptation, and Community Assembly of the American Oaks (Quercus): a Model Clade for Integrating Ecology and Evolution,” by Jeannine Cavender-Bares, in New Phytologist, Vol. 221; 2019 (Florida schematic); Hilary Major (leaves); “Sympatric Parallel Diversification of Major Oak Clades in the Americas and the Origins of Mexican Species Diversity,” by Andrew L. Hipp et al., in New Phytologist, Vol. 217; 2018 (North American phylogeny)
Lands of Opportunity
As temperatures cooled worldwide, the North American climate also became more seasonal. The Rocky Mountains were continuing to rise, and their rain shadow dried out the Great Plains. The tropical forests and broad-leaved evergreen forests that had flourished across North America were gradually restricted in range and driven to extinction by around 40 million years ago. Oak pollen and leaf impressions became more common in the North American fossil record 35 million years ago, by which time decreased temperatures and increased seasonality had converted North America north of Mexico from a mostly tropical to a mostly temperate continental landscape. As climate change extirpated tropical forests from North America, ecological opportunity arose for the oaks.
The red and white oaks moved south into this newly opened territory, each splitting into a lineage on the western side of the Rocky Mountains that gave rise to the modern-day oaks of California and the Pacific Northwest and into a lineage on the eastern side of the Rockies that gave rise to the oaks of eastern North America. Within the latter region, each of these major oak groups subdivided into a predominantly northeastern lineage, a predominantly southeastern lineage and a primarily Texan lineage. From eastern North America, perhaps by way of Texas, the red and white oaks then moved into Mexico between 10 million and 20 million years ago.
In all these areas, palms and broad-leaved evergreen trees had been pushed south or driven partially or wholly extinct by the cooling and increasingly fluctuating climate. The resulting abundance of open habitat enabled oaks to diversify. Increased ecological opportunity allowed oaks to undergo an adaptive radiation, in which nascent species rapidly fill spaces that other species are not occupying. In doing so, these young populations became more ecologically distinct from one another, thereby limiting the movement of genes between them. They became reproductively isolated, so that genes moved less between separated populations than among trees within populations. Subsequently, new genetic mutations and rearrangements could accumulate that distinguished the populations from one another. Through this process, they became new species.
This adaptive radiation played out most dramatically in Mexico and Central America, where about 40 percent of all the world's oaks reside. Recall that oaks were a largely cold-adapted lineage that spread across the continent as temperatures dropped and seasonality increased. As they migrated south into Mexico, oaks climbed to higher elevations that more closely resembled the temperate biome in which they had evolved, and they encountered high topographic variation that readily separated them into reproductively isolated populations. Oaks also evolved more rapidly along the continuum from low water availability to high water availability as they moved into Mexico. Tacking up and down the mountains, different populations adapted to different levels of drought. This ecological differentiation most likely worked hand in hand with increased physical separation to promote reproductive isolation between populations.
Thus, the reason for the high oak diversity in Mexico appears not to be warmer temperatures. And because Mexican oaks are relatively young, their high diversity has not accrued over comparatively long periods of evolutionary time. Rather adaptive radiation led to higher speciation rates in these evolutionarily young Mexican oaks as they moved into the mountains. This change suggests that if oaks had been suited to climb into the Rockies and flourish there—that is, if they could have survived the combination of short growing seasons and cold winters of the northern mountains—they might have developed high diversity in this region as well. Their evolutionary heritage simply did not equip them for these extremely harsh environments. Only a lone white oak species, the Gambel oak (Quercus gambelii), even comes close, and that species is limited to the southern Rockies.
The oaks were finally stopped in their march southward, perhaps by dramatic reduction in seasonality or strong competition from tropical forest species, only barely making it across the Isthmus of Panama into the north of South America. Yet this is not the whole story. The oaks' southward journey actually played out twice, simultaneously and in the same places. Because white and red oaks had already separated from each other by the time they started moving south, this diversification history happened in parallel in both the red and white oaks. Two distinct but very closely related lineages, not one, traced the biogeographical history we just described: moving south, splitting around the Rocky Mountains, heading into Mexico from an eastern North American ancestor. This history may explain part of the species richness and abundance of oaks in the Americas. They essentially double-dipped as they ventured south.

Fossil acorn from Oregon dates to the Eocene epoch. Credit: Thomas J. Bones
Good Neighbors
One of the most exciting areas of our research has been the integration of a genome-level understanding of the oak tree of life with physiological studies of oak adaptation to climate and habitat and community studies of oak forest structure. As oaks spread south and diversified in different regions, the white and the red oaks encountered similar habitats and repeatedly solved the same ecological problems in novel ways. As a result, we often find red and white oaks growing together in the same habitats. For example, on poor rocky soils and bluffs in the eastern U.S., you can find the white oak Quercus stellata, also known as the post oak, growing next to the red oak Quercus marilandica, commonly called the blackjack oak. In the mountains of southern Arizona, the iconic white oak Quercus arizonica often grows beside the red oak Quercus emoryi.
This pattern of oak co-occurrence is found in wooded plant communities across much of the country, and it has another intriguing feature. Whereas distantly related oaks tend to grow together, closely related oaks within lineages tend not to be found together. Along an elevational gradient in the Chiricahua Mountains of southern Arizona, for example, white oak species pass the baton as you walk upslope, transitioning broadly from one to the next as you hike uphill, and red oak species do as well. In the lowlands of Florida, white oak species separate across the sandhill, scrub and ravine habitats shaped by karst topography and fire. Red oaks do the same.
What shapes this pattern of oak co-occurrence? Ecological differentiation within the red and white oaks is influenced in part by the fact that no single species is able to master every habitat. Instead species tend to specialize on a limited part of the available ecological space. In oaks, physiological trade-offs within each lineage subdivide habitat and climatic space so that close relatives are less likely to co-occur. In the Chiricahua Mountains, for instance, drought adaptation separates close relatives along the elevation gradient. Species living near the bottom of the mountain are particularly good at avoiding drought, dropping their leaves during dry seasons. Species living at higher elevation, where there is more overall moisture, focus on surviving daily fluctuations in water availability by allowing leaf water content to drop lower before they suffer damage.
In contrast, in Florida, which is comparatively flat, soil moisture availability and fire intensity structure oak communities. Closely related species in these communities show trade-offs between growth rate and drought tolerance along moisture gradients and between bark thickness and the ability to reproduce via underground stems along gradients of fire intensity. In both regions, and indeed across the country, parallel trade-offs are found in both red and white oaks, and trees with convergent traits from the two lineages tend to grow together.
Members of different lineages may co-exist well with one another in each habitat because they differ in their susceptibility to disease: proximity to a more distantly related neighbor may be less likely to result in an epidemic because red and white oaks tend not to spread the same diseases. There is even evidence that oaks help one another get established and persist by creating a soil environment that benefits the mycorrhizal fungi they need to acquire nutrients. Then, once a forest has become established, oaks become dominant and prevent other kinds of trees from setting up shop. Our work makes clear that the evolutionary origins of oaks shape the complex ecological interactions that help to explain why the trees are so abundant and diverse in North America. The tree of life casts its shadow across the structure of our oak forests.
Creative Hybridization
Now that we can delineate the branching history of the oak tree of life in some detail, the trees' propensity to hybridize has become all the more interesting. People often think of hybridization as a destructive force, eroding genetic differences between species. Yet oaks form what is called a syngameon, in which ecologically and physically distinctive species persist in spite of ongoing gene flow. It has long been hypothesized that genes migrating between species of the syngameon might help oaks adapt to novel environments. Could, for example, genes that contribute to drought adaptation in the post oak migrate into the bur oak (Quercus macrocarpa) in the southern regions, where they co-occur, and help the bur oak adapt to the drying conditions it is expected to encounter under global warming? We know already that there is localized gene flow between oak species and that species differ in what genes they exchange depending on where on the landscape they are, what species they co-occur with, and the climate and habitat in which the trees are growing. We also know that after genes move from one species into the other, they can move beyond the range of the species in which they arose, apparently propelled by environmental selection. These examples suggest that adaptive gene flow may play an important role in oak evolution. We are on the cusp of the integrative genomic and ecological studies needed to understand this process in depth.
We would still like to know what genes and attributes—flowering time, habitat preference, geographical distance—drive speciation in oaks and whether ecological differences evolve while populations are growing together or only when they are separated. We are close to understanding what genes shape differentiation. Recent work in European oaks shows that genes influencing both their ability to cross-pollinate and their ecological preferences (for instance, tolerance of drought, cold and disease) are involved in species differentiation. Yet these findings only tell us that ecological differences evolve in species, not that they drive species differences. Statistical analyses that simulate alternative speciation histories suggest that in a group of four widespread European white oaks that hybridize today, the genomic differences between the species arose when the species were born in different geographical areas, with opportunities for gene flow arising only after the fully formed species migrated back into contact with each other. Still, the high degree of species co-occurrence in the American oaks raises the question of whether hybridization contributed to their diversity.
A firm grasp of when, where and how oaks came to be so diverse is crucial to understanding how oaks will resist and adapt to rapidly changing environments. Oaks migrated rapidly as continental glaciers receded starting around 20,000 years ago, and hybridization between species appears to have been key to their rapid response. The insights we can gain from elucidating the adaptive benefits of gene flow are critical to predicting how resilient oaks may be as climate change exposes them to fungal and insect diseases with which they did not evolve. As insects that transport pathogenic fungi increase their ranges and change their patterns of reproduction with earlier springs, oaks may have trouble holding their ground unless they can evolve quickly enough to resist diseases they have never before encountered. Our challenge for the coming decade as plant biodiversity scientists will be to figure out how differentiation between species and movement of genes between those species will influence the trajectory of oak evolution and population persistence. If we understand these processes well enough, we stand a chance of using that knowledge to predict what our forests will look like a century or more from now. Perhaps it can guide our plans to manage longer-term survival of the vital oaks.

