Late one moonlit night, three fictional revelers on an English moor were transfixed by a horrific sight: “a foul thing, a great, black beast, shaped like a hound, yet larger than any hound that ever mortal eye has rested upon. And even as they looked the thing tore the throat out of Hugo Baskerville, on which, as it turned its eyes and dripping jaws upon them, the three shrieked with fear and rode for dear life.” Historians of medicine have traced the terror that the The Hound of the Baskervilles evoked in Arthur Conan Doyle’s fans to the profound impact of rabies on contemporary British consciousness. With an ability to turn the most placid of pets into frothing, raging beasts and an almost 100 percent mortality rate, the rabies virus was one of the most feared scourges in human history.
As early as 1804, experiments by German physician Georg Gottfried Zinke indicated that the virus occurs in high concentrations in the saliva of an infected animal. The germ also acts to enhance the production of saliva while increasing the amount of it present in the mouth—explaining why rabid dogs drool. Louis Pasteur went on to demonstrate in the 1880s that the brain, too, is infested with the virus. None of this is an accident. Two centuries of research have now established that the rabies virus combines a propensity to be transferred from the saliva-soaked jaws of an infected animal with a diabolical ability to drive it into a frenzy of aggressive biting. By a feat of evolution, the virus manipulates the host’s brain to ensure its own efficient transmission.
Rabies still kills more than 59,000 people annually. Thanks to vaccinations and the quarantine of infected animals, however, it no longer evokes terror in the developed world. Rather neuroscientists are turning the malign germ to the advantage of humankind. The rabies virus is adept at making its way from the site of the bite to the brain by jumping stealthily from neuron to neuron—thereby evading detection by the immune system. A number of researchers, including those in my group at the Sainsbury Wellcome Center for Neural Circuits and Behavior in London, have now harnessed and refined this ability to visualize the connections between neurons.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The human brain consists of billions of neurons, each connected to thousands of others; mapping this tangled web of wires is essential for understanding how it generates our emotions and behaviors. Using engineered varieties of the rabies virus, we can now observe what kinds of inputs a particular type of neuron receives, how electrical signals move from the eye to the brain and what types of neurons control posture to keep us from falling over. Although the field is still in its infancy, in the future such information could help us understand, and perhaps find remedies for, neurological disorders such as Parkinson’s disease.
From bite to brain
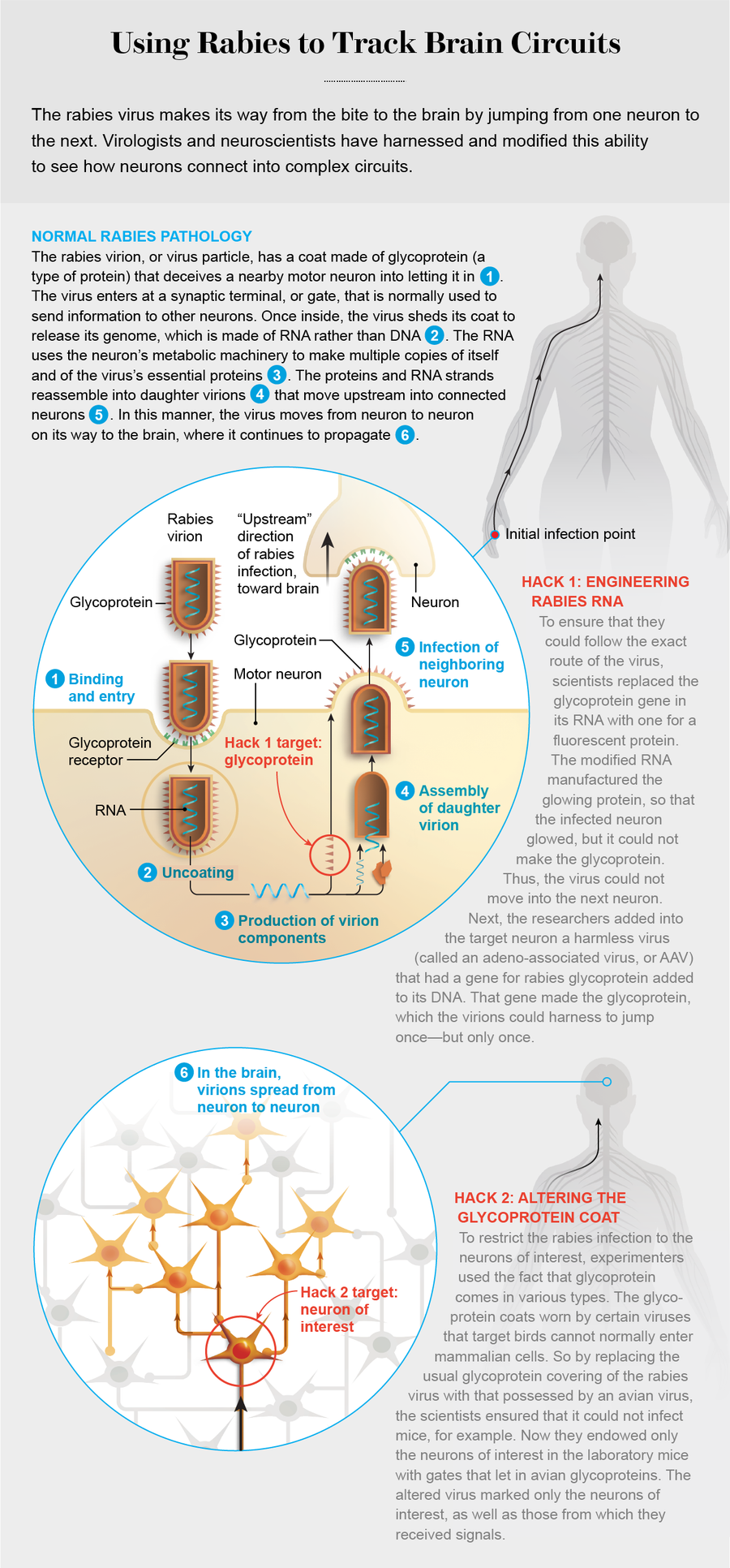
To begin with, the bite injects virions, or virus particles, into muscle tissue. A bullet-shaped capsule containing a single strand of RNA and proteins, the rabies virion is coated with a spiky protein, called a glycoprotein. This coat tricks motor neurons that send projections to the assault site into bringing the virus inside. Motor neurons emit chemicals that cause muscles to contract, and they are linked by a long chain of other neurons to the victim’s brain—the virus’s ultimate destination.
To be precise, the glycoprotein binds to a receptor on a synaptic terminal of the neuron: a point where it transmits signals to a neighboring neuron. Like a door through which one only exits the secure area of an airport—but not enters—the synaptic terminal guards a one-way passage—a synapse—between the neurons. By convention, the “downstream” direction of the synapse is the flow of signals from one neuron to the next, all the way from the brain to the muscles. The rabies virus travels upstream, however, because it has to get to the brain. As such, it fools the receptor to enter a motor neuron through the exit gate.
Viruses are adept at using their host’s cells for their own purposes, but few can beat rabies at the task. Once inside, the intruder throws off its glycoprotein disguise, and its RNA gets to work, using the cell’s materials and metabolism to produce copies of itself, as well as of all its characteristic proteins. These components then reassemble to create daughter virions. Whereas many virus species replicate so rapidly that they force the infected cell to burst open, releasing the virions into the space between the cells, the rabies virus strictly regulates its reproduction—producing just enough daughters to keep moving on. That way, it refrains from causing so much damage that it alerts the immune system. Instead it leaves the host cell intact and crosses a synapse to a new upstream neuron. That sneakiness is one reason the disease has such a long, symptomless incubation period, typically one to three months in humans.
Having thus jumped to a new neuron, the virion starts the entire process again: undressing and copying itself and reassembling daughters that move into the next upstream neuron. In this way, the rabies virus picks a path through the nervous system, creeping from the motor neuron it first encountered in the muscle tissue, through the spinal cord and into the brain.
By the early 2000s several research groups, including those of Gabriella Ugolini, now at the Paris-Saclay Institute of Neuroscience, and Peter Strick, now at the University of Pittsburgh, were pursuing the use of rabies as a tracer for neuronal circuits. Deciphering the route that the virus took from the muscle to the brain was a challenge, however. As a neuroscientist looking at a snapshot of neurons that had been infected with the virus, how could you distinguish between the first jump of the invader from one neuron to the next, the second jump, and so on?
The researchers initially solved this problem by euthanizing laboratory animals shortly after infection, thereby allowing the virus to spread across only one or two synapses. This approach uncovered some of the major pathways in the brain that contribute to motor control. But it had its drawbacks. Not all connections between neurons are equal. A synapse may be strong (or weak), making it more (or less) likely that a signal moving across it will prompt the target neuron to fire in response. Another might be located close to the cell body instead of far away at the end of a projection. And some neurons make a single link with a downstream neuron, whereas others may make hundreds. This heterogeneity means that the virus takes varying lengths of time to travel from one neuron to the next, adding a layer of uncertainty. What if the virus moves through two or three strong synapses before it passes through a weak one?
Viral Engineering
To get around this problem, scientists needed to rejigger the rabies virus. Molecular biologists have developed the amazing ability to manipulate DNA: swapping out genes has become as routine for them as making coffee in the lab kitchen. The wild rabies virus has no DNA to manipulate, however, only RNA. The advent of reverse genetics, which flips the normal genetic cycle by making RNA from DNA, got around that hurdle. In 1994 Matthias Schnell and Karl-Klaus Conzelmann, both then at the Federal Research Center for Virus Diseases of Animals in Tübingen, Germany, produced a functional rabies virus in the lab from cloned DNA alone. They even altered the rabies genome: the RNA string that encodes its characteristic properties.
The ability to manipulate the genome swiftly led to a greater understanding of how the different rabies genes contribute to the virus’s diverse skills. Only one gene was essential to its ability to move between neurons, it turned out: the one that coded for glycoprotein. A rabies virus that had the glycoprotein gene removed from its genome could infect a cell, but once inside it was stuck there. This would be the discovery that thrust the virus into mainstream neuroscience.
In 2007 a collaboration between neuroscientists Ian Wickersham and Edward Callaway, both then at the Salk Institute for Biological Studies in La Jolla, Calif., and virologists Conzelmann and Stefan Finke of the Friedrich Loeffler Institute in Germany resulted in an ingenious system to map neuronal circuits. The first step in their scheme was to swap the glycoprotein gene in the rabies genome with one that coded for a fluorescent protein. The engineered virion could not manufacture glycoproteins; instead its RNA made copies of the fluorescent protein (along with all the other rabies proteins)—so the infected cell shone with a bright color of the experimenters’ choosing.
The second step was to provide glycoprotein in the targeted neuron via some other genetic mechanism. That way, the daughter virions could don glycoprotein coats and jump once—but no more. To that end, the scientists harnessed a very simple type of virus, called an adeno-associated virus (AAV) because it is often found along with much larger viruses called adenoviruses. AAVs contain a tiny amount of DNA. The Salk researchers inserted a gene for making the rabies glycoprotein into that DNA. The rabies virion could harness the glycoprotein the gene manufactured to jump across a single synapse. It could not, however, take the glycoprotein gene with it because it was a segment of DNA, not of RNA. So when the virion had jumped into the next cell, it was stuck again. At that point, a glance at the infected animal’s brain revealed populations of glowing cells across the nervous system that were directly connected to any neuron researchers wanted to target.
There remained one problem, however. Injection of the rabies virus into the brain resulted in the direct infection of any neuron that sent a projection into the injection site. Without a way to restrict the initial infection of the rabies virus to particular neurons, scientists could not differentiate between neurons that were infected directly by the injected virus and those that were infected after the virus had moved across a synapse. The solution would come from another area of virology: viruses that specifically affect birds.
In the wild, entire classes of viruses can be found that infect only certain groups of animals. For example, the avian sarcoma leukosis virus (ASLV) usually leads to cancer in chickens but cannot normally infect mammalian cells. Like rabies, this virus has a glycoprotein envelope, which comes in a variety of configurations. Different ASLV glycoproteins are known as Env (for envelope), followed by a label for the particular form. Each subtype binds to a specific receptor. So, for example, EnvA binds to a receptor called TVA (for avian tumor receptor virus A). If a cell does not possess the TVA receptor, it cannot be infected with an EnvA-coated virus. This selective interaction enables researchers to restrict the initial infection of rabies virus to one type of neuron.
By introducing the gene for EnvA glycoprotein in a rabies-infested cell culture (a process known as pseudotyping), Wickersham, Callaway and their colleagues replaced the native glycoprotein coat on the rabies virus with the EnvA glycoprotein from the avian virus. Thus altered, the rabies virus could not deceive any mammalian cells into letting it in. By endowing the neuron of interest, typically in a mouse brain, with the TVA receptor, neuroscientists could be assured that the rabies virus would infect only this cell.
The target neuron (in practice, a class of neurons) was also supplied with an AAV containing the gene for rabies glycoprotein. Once inside, the rabies virus shed its chicken costume, picked up its normal cloak and jumped into upstream neurons. By engineering the rabies virus to infect—and hop only once from—a well-defined group of “starter” neurons, researchers could now get a clear image of how the brain was wired.
Tuning rabies
The simplicity and elegance of the delta-G rabies system (as its inventors called it because of the altered glycoprotein) took the neuroscience community by storm. Using it, researchers could see right away what kinds of neurons send signals to the neurons of interest. Like all new technologies, however, the scheme had its imperfections. Sometimes the number of connections labeled were rather small—on the order of 10 per starter neuron.
Credit: Kelly Murphy
Around 2015 Thomas Reardon, Thomas Jessell, Attila Losonczy and I, all then at Columbia University, were using the delta-G system to understand the neural circuits that guided motor commands. Finding relatively low numbers of connections to motor neurons in the spinal cord or the brain, we suspected we were getting an incomplete picture of the circuitry. Another issue was neurotoxicity. Once the virus was in a cell, it would start to break down and die within a couple of weeks. If the virus itself was causing individual neurons to alter their behavior, interpreting any observations could be problematic.
Schnell and Christoph Wirblich, both at Thomas Jefferson University, had done pioneering work on rabies virus biology, so we went to them for help. They knew right away that our problems stemmed from the strain of virus that we were using. It had originally been developed for use in a rabies vaccine. Vaccines incorporate special strains of the germ that humans have selected to reproduce unusually rapidly so that the multitudinous daughter virions explode out of the infected cells and alert the immune system before it is too late. That indicated a way to refine our research tool. Because we were using mice in our studies, our virologist collaborators suggested that we instead try a strain that had been tuned over many years to infect mouse neurons.
The parent virus of this strain had originally been isolated in the wild and then “fixed” in the lab by being repeatedly passed through the brains of mice or through cell lines. It had thereby evolved to be a specialist at targeting the mouse nervous system. After assembling a neuronal tracing mechanism based on this mouse-specific strain, we found that it labeled many more connections than we had previously seen. Moreover, being an expert at evading the mouse immune system, it made relatively small amounts of each protein. As such, it placed less strain on the host cell’s machinery and allowed neurons to remain relatively healthy.
We further altered our tracing system to replace the gene for the fluorescent protein in the rabies virus with one for a light-sensitive protein, called channelrhodopsin (ChR), originally found in green algae. When activated by blue light, this remarkable molecule opened a channel that allowed positively charged ions to flow into the target neuron, prompting it to emit an electrical signal. (The infected cell continued to glow, however, because we used a version of ChR that included a fluorescent protein.) With this fine-tuned rabies virus system, we could watch entire neuronal circuits fire during certain actions of the mouse or switch them on or off—for up to a month after the virus had infected a neuron. That gave us ample time to conduct many of the tests we needed to understand how specific circuits generate behavior.
Wiring Diagram
Using different versions of the delta-G rabies system, neuroscientists have probed many different circuits in the nervous system to understand how they contribute to the perceptions and behaviors of animals. Take, for instance, the visual system. When light enters the eye, neurons at the back of the retina, called retinal ganglion cells, transmit signals to the brain. Neuroscientists traditionally believed that this information travels to intermediate locations in the brain, ultimately ending up in the cerebral cortex—the celebrated gray matter—where it is processed. Botond Roska’s group at the Friedrich Miescher Institute for Biomedical Research in Switzerland used the rabies system to trace the inputs from the retinal ganglion cells to the lateral geniculate nucleus (LGN), an area of the brain that was regarded as just another relay to the cortex.
The researchers demonstrated that the LGN contained three different types of neurons, each likely processing visual information differently. Indeed, less than a third of the neurons served as a relay, providing a direct line from the retina to the cortex. But roughly another third received combinations of different inputs from one eye; the remaining neurons (about 40 percent) got signals from both eyes. Thus, although the LGN lies at an early stage of the visual circuit, most of its neurons integrate information from multiple sources. The finding will likely illuminate the process by which the brain interprets information from the eyes.
At Columbia, my co-workers and I investigated the neurons in the lateral vestibular nucleus (LVN), a brain region that tries to prevent us from falling over. Imagine being on a moving subway train that stops unexpectedly. Before you have had time to think, you shift your feet to compensate, stiffen your legs and perhaps grab the nearest pole. How does the brain activate the right groups of muscles so swiftly in a variety of similar situations?
We found that the LVN of mice contains two anatomically distinct types of neurons, each having different downstream connections to parts of the nervous system. One group switches on very quickly after your brain senses your body is unstable; these neurons act to extend the limbs to widen the base of support. Later, a second set of LVN neurons become active. These serve to strengthen and stabilize the joints in the same limb, enabling the body to be pushed back to its original position. We could activate these neurons simply by switching on a blue light, delivered to the LVN by a fiber-optic cable. When the light came on, the mice adjusted the positions of their limbs, as if to stop themselves from falling over—even when they were not off-balance.
Nao Uchida’s lab at Harvard University investigated a third significant question: What are the functions of neurons that release dopamine? Such “dopaminergic” neurons in two regions of the brain, the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA), have long been known to respond to rewards. They would become very excited when a test animal got a treat or when a sensory stimulus predicted that it was about to come. (Think of eating a candy bar, compared with hearing the rustling of its wrapper.) To understand what types of information the neurons were receiving, scientists needed to know how they were connected to other brain circuits. Using the delta-G system, the Harvard team found that dopaminergic neurons in the SNc received information about the relevance of a stimulus: Is this sound of a candy wrapper going to get me a piece of chocolate? In contrast, the VTA received information on the quality of the reward: How good is this candy?
As it happens, dopaminergic neurons in the SNc degenerate in Parkinson’s. Intriguingly, Uchida and his colleagues also discovered that major inputs into such neurons in the SNc come from the subthalamic nucleus, a small, lens-shaped region of the brain that, along with similar nuclei, is involved in controlling movement. Exciting the subthalamic nucleus by means of an inserted electrode, in a technique known as deep-brain stimulation, is often effective at relieving symptoms of Parkinson’s. Surmising that the inputs they had discovered explained why such stimulation works, the neuroscientists reasoned that targeting other brain regions, which they had identified as also sending inputs to the SNc, might aid some Parkinson’s patients.
The combination of natural evolution and targeted engineering has thus given neuroscientists a remarkably powerful tool. There is still much room for improvement. For example, will it be possible to engineer viruses that move downstream, labeling a neuron’s outputs instead of its inputs? Can we make a virus that labels only active connections between neurons, lighting up the circuits that are involved in distinct behaviors? The time has come for a virus that has manipulated and terrorized humans for millennia to be manipulated to serve us.