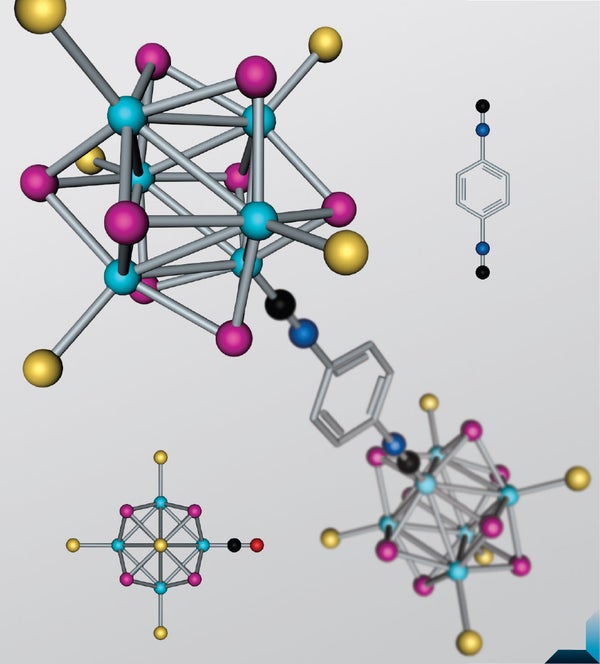
The periodic table may look like it has plenty of elements, but chemists and materials scientists would like more, thank you. That is because in the quest to design synthetic materials with unusually useful properties—say, a siliconlike superconductor with the biodegradability of wood—nature's cookbook has its limits. “Oftentimes you'll want an atom that actually doesn't exist,” says Colin Nuckolls, a professor of chemistry at Columbia University. Molecules built out of so-called superatoms—clusters of atoms that behave like single elemental units—could fill this need. Superatoms can be given electronic and magnetic properties that would be difficult or impossible to achieve using natural combinations of elements. But although chemists have known for decades how to create superatoms, a reliable way of linking them into larger structures has proved elusive.
Now Nuckolls's team has discovered a method for constructing “designer molecules” out of superatoms. These synthetic structures could mimic the properties of naturally occurring molecules while giving materials scientists the ability to “tune” their properties to match particular purposes. “You can easily vary chemical or magnetic properties of superatom molecules in ways you wouldn't be able to do with just atomic structures,” Nuckolls says. “It's like adding another dimension to the periodic table.”
Walter Knight and his collaborators at the University of California, Berkeley, discovered superatoms in 1984 by synthesizing clusters of sodium atoms whose outer shell of electrons behaved like the shell of a single atom, enhancing magnetic and reactive properties. Since then, scientists have made superatomic clusters from aluminum, platinum, rubidium and other elements. But to combine superatoms into larger molecules, scientists needed to figure out the special chemical rules that superatoms follow, which differ from those of their cousins in the periodic table.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Electrons naturally arrange themselves around the nucleus of an atom in an orderly fashion known as Aufbau, occupying lower energy levels before higher ones. (Aufbau means “building up” in German; the principle was introduced in the early years of quantum mechanics.) Based on an initial discovery by graduate student Anouck M. Champsaur, Nuckolls's team pieced together the beginnings of an Aufbau-like principle for making synthetic molecules out of superatoms.
So far the team has constructed molecules from pairs and trios of cobalt-selenium superatoms. But Champsaur and Nuckolls believe that a superatom Aufbau will enable the synthesis of more exotic materials with potential applications in flexible sensors, smart clothing and high-efficiency batteries. Chemistry textbooks won't need to update their periodic tables, Nuckolls says: “That would be alchemy.” But superatom molecules, he says, are “a way of getting more than nature gave you.”